Purpose
According to the International Federation of Gynecology and Obstetrics (FIGO), the recurrence rates of patients with stage IB, IIA, IIB, III, and IVA cervical cancer, who received radiotherapy alone are 10%, 17%, 23%, 42%, and 74%, respectively [1, 2]. If cervical cancer recurs after treatment, the likelihood of receiving treatment again is low. Prognosis of patients with recurrent cervical cancer has been reported to be poor, with one-year survival rates ranging from 15% to 20%, and five-year survival rates ranging from 3.2% to 16.5% [3]. The extent of lesion, mode of initial treatment, and characteristics of recurrent lesions all influence the treatment and prognosis of recurrent cervical cancer. Radiation therapy can be applied to localized pelvic recurrence after initial surgical treatment, which makes re-operation difficult and risky due to radiotherapy-induced fibrosis of the lesion. Overall prognosis of patients with metastatic cervical cancer with a localized recurrence are very poor, and the choice of treatment option poses a considerable clinical challenge [4, 5].
The treatment for pelvic recurrence of cervical cancer after radiotherapy routinely include 3 main modalities: surgery, radiotherapy, and chemotherapy. For patients with central pelvic recurrence after radiotherapy without pelvic wall invasion and no distant metastasis, pelvic exenteration can be chosen, but surgery is difficult to perform, and has a high incidence of post-operative complications. The incidence of peri-operative complications ranges from 16% to 71%, the incidence of late complications ranges from 36% to 61%, and the intra-operative and post-operative mortality rate ranges from 0% to 12% [6]. In patients with pelvic wall recurrence after radiotherapy, pelvic exenteration usually fails to achieve cure, and extended medial pelvic wall resection combined with intra-operative radiotherapy can benefit patients with no meatus residuals, but severe intestinal and neurologic adverse effects occur in 25% to 30% of patients. Chemotherapy is used essentially as a palliative treatment for patients with recurrent, metastatic, or persistent cervical cancer.
In recent years, radioactive particle therapy has been widely used in treatment of various recurrent solid tumors due to its advantages of high-dose delivered to local lesion, low-dose to adjacent normal tissues, and minimally invasive nature of the procedure [7-9], thus providing an effective curative treatment for patients with locally recurrent cervical cancer in the pelvis. Using interstitial brachytherapy of iodine-125 (125I) radioactive seeds in recurrent pelvic tumor under the guidance of high-resolution computed tomography scanner can significantly increase therapeutic dose delivered to tumor target and reduce probability of unnecessary damage to surrounding normal tissues. In this paper, we retrospectively analyzed the clinical efficacy of 46 patients with locally recurrent cervical cancer in the pelvis after radiotherapy between June 2016 and July 2022 treated with 125I particles.
Material and methods
Research object
Sixty cervical cancer patients, who were treated with radioactive 125I particle implantation from June 2016 to July 2022 were selected. All 60 patients had localized pelvic recurrence after radiotherapy (Fig. 1), and 46 patients with pelvic local recurrence were analyzed. Tumor staging was based on cervical cancer staging criteria established by the International Society of Oncology and the Federation International of Gynecology and Obstetrics (FIGO): 18 cases of stage IIA, 11 cases of stage IIB, 4 cases of stage IIIA, and 13 cases of stage IIIB. General status of patients was evaluated according to Karnofsky performance status (KPS), and the KPS score was ≥ 70. All localized pelvic recurrence sites had received prior external radiation therapy, of which 19.57% (9/46) had received re-treatment radiotherapy, with a median cumulative radiotherapy dose of 56 (range, 43-107) Gy. Forty-six patients had localized pelvic pain or bilateral lower extremity pressure pain, with mild pain in 15 cases, moderate pain in 26 cases, and severe pain in 5 cases. Median progression-free survival (PFS) time after prior treatment was 14.6 (range, 6-58) months. Patients’ characteristics are provided in Table 1.
Table 1
Clinical characteristics
Instrumentals and equipment
Computer tomography was done with 64-row CT routine scanning configured by Discovery VCT PET/CT (GE, USA). Required particle implantation instruments, including implantation guns and push thimbles were produced by Zhejiang Xiangshan, and puncture needles were 15-20 cm × 18 G (Hakko, Japan). Radiotherapy treatment planning system (TPS) system was provided by Zhuhai Hejia Medical Equipment Co. (HGGR-2000 version). Particle source was supplied by Beijing Atomic Hi-Tech Co., Ltd., with particle diameter of 0.8 mm and length of 4.5 mm. The shell was titanium metal fully closed, and the particle activity was between 0.4-0.8 mCi, with half-life of 60 days. Particles were sterilized by high temperature using high pressure steam sterilization method. Serum squamous cell carcinoma-associated antigen (SCC-Ag) detection was determined by chemiluminescence (Shenzhen New Industry Biological Co.) Serum carcinoembryonic antigen (CEA) was measured with chemiluminescence (Soling-LIAISON chemiluminescence instrument, kit provided by Soling-LIAISON, reference range of 0-5 ng/ml).
Methods
Pre-operative procedure
Before surgery, all patients received physical examination, blood routine test, coagulation function, liver and kidney function, urine routine test, SCC-Ag, CEA [10] and quantitative C-reactive protein tests, gynecological examination, electrocardiogram, chest radiographs, and whole abdomen and pelvic enhancement CT. All patients signed an informed consent for radioactive particle implantation surgery.
Operative procedure
Enhanced CT images were imported into TPS, which predicted the number of particles to be implanted, particles’ distribution, and total dose to be used. Moreover, it developed a specific puncture plan, selected the puncture point, and designed the route of needle insertion. Based on location of pre-punctured lesion, patient’s condition, and actual need for puncture, the patient was assisted in selecting appropriate puncture position. A positioning grating was placed in the skin area corresponding to pre-punctured lesion. CT scan was performed to locate the puncture point, to determine the angle and depth of puncture needle, and local anesthesia of 2% lidocaine was injected into the skin; the needle was laid out layer by layer according to TPS plan. During particle implantation, it is important to avoid nerves and blood vessels, and cardiac monitoring during implantation process as well as observation of the patient’s consciousness, pain, respiration, bleeding, etc.
Post-operative verification
After completion of the surgery, CT scans should be done again immediately to rule out bleeding and other complications. The average number of particles implanted in the 57 lesions was 24.8 (range, 8-42), with no statistically significant difference compared with pre-operative TPS plan (p > 0.05). The median particle activity was 22.6 (range, 14.8-29.9) MBq, and the median 90% gross tumor volume (GTV) dose delivered to 90% GTV (D90%) was 91.5 (range, 49.5-120) Gy. After surgery, CT images were inputted into TPS for post-implant dosimetry, so that GTV D90% (dose delivered to 90% GTV) was as close as possible to the prescribed dose; critical structures were located outside the 1 cm range of isodose line of the prescribed dose.
Follow-up visits
Post-operative follow-up visits were performed every 3 months, and included monitoring of patients’ clinical symptoms, imaging examinations, and serum tumor markers. According to the Reflective Evaluation Criteria for Solid Tumor Treatment (RECIST) [11], complete remission (CR), partial remission (PR), stable disease (SD), progressive disease (PD) were assessed. Local control rate measured was CR + PR + SD × 100% and effective rate was CR + PR × 100%. According to an expert consensus proposed by the Expert Committee on Gynecologic Oncology Laboratory Medicine of the Chinese Medical Doctors’ Association [10], the changes of serum tumor markers SCC-Ag and CEA before and 1 month after 125I particle therapy were monitored. At the same time, patients’ pain relief was evaluated at 1 month after the procedure. Evaluation of pain efficacy referred to the WHO’s malignant tumor efficacy evaluation standard, where obvious effect was formulated as the disappearance of pain or decrease of pain grading by 2 grades, effective effect was expressed as the decrease of pain grading by 1 grade, and ineffective effect was formulated as the absence of decrease or the increase of pain grading. Adverse effects were evaluated according to the RTOG/EORTC late radiation injury grading scheme.
Statistical analysis
SPSS 21.0 software was used to analyze data, and measurement data were expressed as mean ± standard deviation (x ±s) by t-test. Count data were expressed as rate (%) by χ2 test, local control rate of locally recurrent foci in the pelvis of cervical cancer was calculated with Kaplan-Meier method, and analysis was carried using log-rank test for single-factor and Cox regression model for multi-factor. P < 0.05 was set as statistically significant, with 0.05 considered statistically significant difference.
Results
Evaluation of efficacy
CT review of 57 lesions was performed 3 months after surgery, and according to the efficacy evaluation criteria of CR 8, PR 31, SD 11, and PD 7, the local control rate was 87.72% (50/57) and the effective rate was 68.42% (39/57). Upon further analysis, the local control rate of patients in stages II and III was 86.21% (25/29) and 82.35% (14/17), respectively, with no statistically significant difference (χ2 = 0.494, p = 0.256 > 0.05), and the effective rate of the two stages was 79.31% (23/29) and 47.06% (8/17), respectively, with a statistically significant difference (χ2 = 10.862, p < 0.001). The efficacy of each pathologic stage is shown in Table 2.
Pain improvement
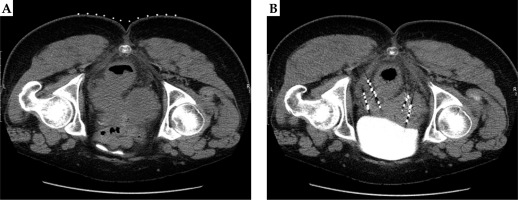
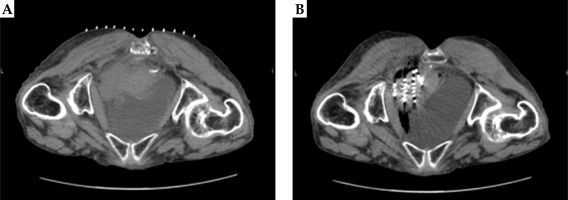
All 46 patients were accompanied by pain, of which 35 had pain relief at the end of the treatment, with an effective rate of pain relief of 76.09%. Pain evaluation was performed again 1 month after the surgery, and 42 cases had a pain relief, with an effective rate of pain relief of 91.30%. One patient experienced aggravated pain compared with previously measured level, and 3 patients had pain unrelieved. Also, there was one newly detected pelvic lymph node metastasis in a person with aggravated pain. Before and after images of two patients with locally recurrent cervical cancer in the pelvis after radiotherapy treated with radioactive 125I particles are shown in Figures 2A, B and 3A, B.
Adverse events
During particle implantation, the most common adverse reaction was transient pain exacerbation (n = 12, 26.09%), which was considered to be related with local tissue swelling and compression of the surrounding tissues after puncturing the lesion. Local numbness occurred in 13.04% (6/46) of patients, and was relieved by an adjustment of implantation needle position.
After particle implantation, the most of adverse reactions were grade 1 or 2, with an incidence rate of 36.96%, and no patient experienced grade 3 or above adverse reactions. 28.26% (13/46) of the patients had low-grade fever, with temperature fluctuating from 37.5°C to 38.6°C. Nine patients recovered to normal after physical hypothermia treatment, and four patients improved after symptomatic treatment. 19.57% (9/46) of the patients developed mild or moderate abdominal pain after treatment, which was relieved after application of pain medication; 8.70% (4/46) patients developed grade 1 or 2 proctitis, 4.35% (2/46) patients experienced acute nausea, which was improved after symptomatic treatment, and 4.35% (2/46) patients developed grade 1 urinary reactions. The incidence of particle detachment was 6.52% (3/46), and in these 3 patients, central recurrent trans-vaginal detachment occurred. No complications, such as hemorrhage, needle tract implantation metastasis, enterocutaneous fistula, ureterocutaneous fistula, vesicovaginal fistula, pelvic abscess, intestinal infection, and nerve injury were observed. Adverse events associated with 125I particle implantation are shown in Table 3.
Table 3
Side effects associated with radioactive 125I seed implantation
Survival period
The end of follow-up was May 31, 2023; 14 patients (30.43%) died from non-neoplastic diseases, 29 patients (63.04%) died from tumor metastasis, and 3 patients (6.52%) survived during the follow-up period. The median local progression-free survival (LPFS) time was 12.2 (range, 3.5-32) months, the median overall survival (OS) time was 16.3 (range, 3.5-40) months, and the 1-year and 2-year OS were 63.04% and 41.30%, respectively.
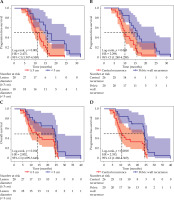
The uni-factorial and multi-factorial analyses are shown in Table 4. The results showed that local recurrent mass size and recurrence site were the main factors affecting survival, and relative hazards (HR) were 2.002 (p = 0.014 < 0.05) and 2.353 (p = 0.0018 < 0.05). According to the analysis of lesion size and recurrence site, the median LPFS was 15.5 months and the median OS was 24.35 months in cases with recurrent mass diameter < 3 cm; the median LPFS was 11.25 months (χ2 = 10.83, p = 0.001 < 0.05) in cases with mass ≥ 3 cm (Fig. 4) and the median OS was 13.85 months (χ2 = 5.986, p = 0.014 < 0.05). Twenty patients with pelvic wall recurrence and 26 patients with central recurrence had the median LPFS of 15.80 and 10.00 months, respectively (χ2 = 8.833, p = 0.0030 < 0.05), and the median OS were 19.25 and 16.45 months, respectively (χ2 = 1.701, p = 0.056 > 0.05). Nine (45.00%) of the 20 patients with pelvic wall recurrence died from non-neoplastic disease, 9 (45.00%) died from tumor metastasis, and 2 (10.00%) survived during the follow-up period. Five (19.23%) of the 26 patients with central recurrence died from non-neoplastic disease, 20 (76.92%) died from tumor metastasis, and 1 (3.85%) survived. Upon further analysis, the median LPFS of the 12 patients with central-type recurrence < 3 cm and the 14 patients with pelvic wall recurrence ≥ 3 cm were 14.00 and 13.00 months, respectively (χ2 = 0.1314, p = 0.7170 > 0.05), and the median OS were 16.00 and 23.50 months, respectively (χ2 = 0.3750, p = 0.5403 > 0.05). Kaplan-Meier curve is shown in Figure 4.
Table 4
Univariate and multivariate analysis of influencing factors associated with localized control rates
Fig. 4
Kaplan-Meier curve. A) Comparison of PFS survival curves in patients with recurrent mass diameter ≥ 3 cm (n = 28), recurrent mass diameter < 3 cm (n = 18) (χ2 = 10.83, p = 0.001). B) Comparison of PFS survival curves in patients with pelvic wall recurrence (n = 20) and central recurrence (n = 26) (χ2 = 8.833, p = 0.0030). C) Comparison of OS survival curves in patients with recurrent mass diameter ≥ 3 cm (n = 28) and recurrent mass diameter < 3 cm (n = 18) (χ2 = 5.986, p = 0.014). D) Comparison of PFS survival curves in patients with pelvic wall recurrence (n = 20) and central recurrence (n = 26) (χ2 = 9.701, p = 0.0018)
Serum tumor marker
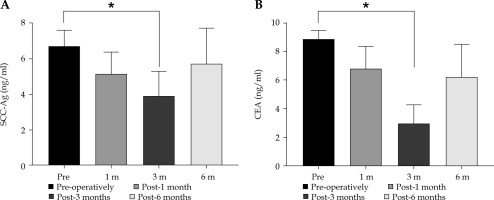
Comparison of SCC-Ag CEA before and after treatment is shown in Figure 5. To further study the relationship between SCC-Ag and patients’ prognosis, the median LPFS of 15.5 months in the pre-treatment SCC-Ag ≤ 3.5 ng/ml group was significantly higher than the median LPFS of 10.2 months in the SCC-Ag > 3.5 ng/ml group, and the difference between these two comparisons was statistically significant (χ2 = 4.241, p = 0.036 < 0.05). Statistics of serum SCC-Ag levels in patients with different clinical stages, SCC-Ag 8.02 ±0.98 ng/ml was significantly higher in patients with stage III (n = 17) than in patients with stage II (n = 29), 5.98 ±0.87 ng/ml before treatment, and the difference was statistically significant (χ2 = 5.012, p = 0.041 < 0.05). Serum SCC-Ag levels of stage II and III patients after treatment were 3.47 ±0.64 ng/ml and 4.26 ±1.22 ng/ml, respectively, and the difference was not statistically significant (χ2 = 1.036, p = 0.081 > 0.05).
Fig. 5
A) Comparison of SCC-Ag/CEA levels between pre-treatment and post-treatment (x ± s). Abscissa represents patients after treatment, and ordinate represents tumor marker level. SCC-Ag level was 6.75 ±0.86 ng/ml pre-operative, and 3.92 ±1.39 ng/ml 3-month post-operative. * Indicate significant difference in SCC-Ag level between pre-treatment and post-treatment (t = 11.74, p < 0.01). B) CEA level was 8.86 ±0.61 ng/ml pre-operative, and 2.98 ±1.27 ng/ml 3-month post-operative. * Indicate significant difference in CEA level between pre-treatment and post-treatment (t = 28.31, p < 0.01)
Discussion
Early clinical manifestations of cervical cancer can appear in the form of vaginal bleeding, and late stage results from tumor invasion, peripheral compression, and other related symptoms. In some patients, hemorrhage can be life-threatening condition, and many patients have a high recurrence rate and poor prognosis despite active treatment. Studies have shown that the 1-year survival rate of post-operative recurrence of cervical cancer is less than 20% [12], and surgical resection is usually chosen again for isolated recurrent lesions in addition to radiotherapy. However, for recurrent cervical cancer patient without indication for re-surgery, who already received adequate and full chemotherapy and radiotherapy [13], clinical treatment is complicated, and it is difficult to provide adequate radiation dose for re-treatment with extra-curricular radiotherapy.
Radioactive 125I seed implantation is a kind of interstitial low-dose-rate brachytherapy, where the radioactive source is placed within or in close proximity to the tumor. Low-energy γ-rays, are released continuously to destroy the DNA structure of the tumor, hence resulting in cells’ death [14, 15]. The steep dose fall-off in brachytherapy is its advantage when compared with rapid dose fall-off in external beam therapy. Relative to external irradiation, radioactive particle implantation has certain benefits. Firstly, its penetration is weaker, the damage to surrounding normal tissues and organs is reduced with attenuation of rays, which can sufficiently improve local dose of the target lesion and reduce radiation dose to surrounding normal tissues. Secondly, the continuous and slow irradiation of rays reduces the re-proliferation of tumors, and once particles are implanted in the target area, they will not change position during movement of irradiated organs. Furthermore, several papers have reported that radioactive particle implantation for solid tumors has clear efficacy, minimally invasive puncture is less painful, and effectively reduces complications. Also, it is less damaging to healthcare workers and nursing family members. Okazawa et al. [16] retrospectively analyzed 15 patients with pelvic central recurrent cervical cancer treated with particle implantation. The 2-year local control and OS rates were 33% and 64%, respectively; 10 cases had a central recurrence after a median of 12.5 (range, 2.5-49.7) months, and 2 patients experienced grade III or IV late complications. This study concluded that permanent implantation of particles could be a therapeutic option for patients with pelvic recurrence of gynecologic tumors, with better efficacy for tumors with vaginal walls or stumps < 2 cm. Tong et al. [17] used CT-guided 125I particle implantation to treat patients with recurrent cervical cancer, and their results demonstrated that the local control rate at 3 months was 84.5%, and the 1-year survival rate was 65.5%.
In the present study, the recent local control rate was 87.72% (50/57), and the effective rate was 68.42% (39/57) for a total of 57 lesions in 46 patients. Moreover, the effective rates for initial clinical stage II (n = 29) and III (n = 17) were 79.31% (23/29) and 47.06% (8/17), respectively, and the differences were statistically significant (χ2 = 10.862, p < 0.001). The pain relief rate was 76.19%.
Radioactive particle dose distribution depends on activity and spatial distribution of the particles, and spatial distribution of the particles depends, to a large extent, on the science of insertion of the implantation needle and distribution of reasonable space (spacing, depth, angle, etc.). Qu et al. [18] studied the dosimetry of radioactive particle implantation therapy in 30 patients with pelvic recurrent cervical cancer after radiotherapy, and concluded that their 1-year and 2-year LPFS rates were 39.4% and 22.5%, respectively, and their 1-year and 2-year OS rates were 57.3% and 27.4%, respectively. This can be prolonged, when D90 ≥ 105 Gy, D100 ≥ 55 Gy, or V100 ≥ 91% LPFS, and D100 is an independent factor affecting LPFS. The LPFS time in this study was 12.2 (range, 3.5-32) months, and the 1-year and 2-year OS were 63.04% and 41.30%, respectively. The median OS time was 16.3 (range, 3.5-40) months, which was similar to the above findings.
In the current study, we used the particle activity of 14.8-29.9 (median, 22.6) MBq, and performed a post-operative dosimetry validation for calculating the coverage of 90% of the target volume at the mean dose of D90, to ensure that the D90% was as close as possible to the prescribed dose and that the endangered organs were located outside the 1 cm range of the isodose line of the prescribed dose.
Analyzing the factors affecting patients’ survival, according to the uni-factorial and multi-factorial analyses suggesting that the size of lesion and the site of recurrence were the main factors, the HR was 2.002 (p = 0.014 < 0.05) and 2.353 (p = 0.0018 < 0.05), respectively. Several papers have reported that the size of lesion is a factor affecting survival of cervical cancer patients [19, 20]. In the present study, we also concluded that the median LPFS was 15.5 months and the median OS was 24.35 months in cases with recurrent mass diameter < 3 cm. The median LPFS was 11.25 months and the median OS was 13.85 months in cases with recurrent mass diameter ≥ 3 cm, with p < 0.05 in both cases. In the analysis of local progression-free survival, pelvic wall recurrence was longer than central recurrence, which may be related to the fact that lesions of central recurrence are easy to spread along the vaginal wall and are not easy to determine with the tumor boundary [21]. However, the difference in overall survival between patients with pelvic wall recurrence and those with central recurrence was not statistically significant, which may be related to the cause of patient’s death: 45% of the 9 patients with pelvic wall recurrence died from non-tumor-related diseases during follow-up period, which reduced the overall survival of patients in the pelvic wall recurrence group.
The Expert Committee of Gynecologic Oncology Laboratory Medicine of the Chinese Medical Doctors’ Association proposed an expert consensus [10]: serum SCC-Ag can not only assist in cervical cancer screening, prognostic assessment, and its efficacy monitoring, but also be used in the recurrence monitoring of cervical cancer. Serum CEA can be applied to the whole treatment stage of cervical cancer, including early screening, differential diagnosis, disease monitoring, and prognostic assessment, but its specificity is poor and needs to be combined with other markers. Squamous cell cervical cancer accounts for 85-90% of all cervical cancers, therefore, this study investigated the level of serum tumor markers, with main monitoring of the changes of SCC-Ag and CEA before and after treatment with 125I particles. SCC-Ag and CEA were significantly decreased compared with pre-treatment measurements (t = 28.31, p < 0.01). Focusing on further study of the relationship between SCC-Ag and patients’ prognosis, the median LPFS in the group with SCC-Ag ≤ 3.5 ng/ml before treatment was significantly higher than that in the group with SCC-Ag > 3.5 ng/ml. The levels of serum SCC-Ag in patients with different clinical stages were significantly higher in patients with stage III than in stage II before treatment (χ2 = 5.012, p = 0.041 < 0.05), and the difference in SCC-Ag between patients with stage II and stage III after treatment was not statistically significant (χ2 = 1.036, p = 0.081 > 0.05). It can be seen that 125I particle implantation for the treatment of locally recurrent cervical cancer in the pelvis after radiotherapy can significantly reduce the level of serum tumor markers. Moreover, SCC-Ag has its value in the assessment of efficacy of cervical cancer treatment and the evaluation of survival prognosis.
During the follow-up period in this study, no serious complications, such as hemorrhage, needle tract implantation metastasis, intestinal fistula, ureteral fistula, bladder fistula, pelvic abscess, intestinal infection, nerve injury, etc., occurred in the 46 patients, and the only adverse reactions seen included transient localized pain, numbness, and related grade 1 or 2, which improved after active symptomatic treatment, indicating that radioactive 125I particle implantation is a safe and well-tolerated method of treatment.
Our findings should be considered in the context of limitations of this study. This was a retrospective study with a small sample size. Larger sample sizes are needed to confirm our results. Further studies should also investigate the optimal dose of 125I brachytherapy and the clinical benefit of treatment regarding prevention of adverse events, progression-free survival, and overall survival.
In conclusion, the use of CT-guided percutaneous puncture radioactive 125I particle implantation for the treatment of pelvic local recurrence of cervical cancer after radiotherapy has the advantages of significant near-term efficacy, less invasiveness, and fewer complications. It can effectively alleviate the symptoms of pain and lower limb edema and significantly reduce the level of serum tumor markers.
In the current study, for patients with initial clinical stage II, the effective rate can be significantly improved, especially for those with local recurrence mass < 3 cm and pelvic wall recurrence, prolonging the survival time of patients. The LPFS of patients with central recurrence < 3 cm is longer than that of patients with pelvic wall recurrence ≥ 3 cm, but due to small number of cases in this study and short follow-up efficacy time, it is not enough to confirm the conclusion. Further study is needed with increased sample size and longer follow-up time to confirm the efficacy.