Summary
This study explores the feasibility of using 3D printing technology to create physical models of paravalvular leaks (PVL) based on data obtained from transesophageal echocardiography (TEE) examinations. PVL closure through transcatheter procedures presents challenges due to complex anatomy and potential interference between implanted occluders and nearby structures. The research aims to enhance procedural planning for structural heart interventions by generating 3D-printed models from TEE data.
Introduction
The increasing number of valve replacement procedures (both transcatheter and surgical) results in a rising number of paravalvular leaks (PVL). The exact incidence rate of PVLs remains unknown, but it is estimated at about 10% of patients after aortic valve surgery and, according to the literature, can even be as high as 15% after mitral valve replacement [1]. Between one and five percent of patients with a PVL are symptomatic and may require invasive treatment [2]. The main signs and symptoms arise from congestive heart failure (90%) and may also be accompanied by hemolysis, occurring in nearly 33–75% of cases [3, 4]. Moreover, PVLs are considered a risk factor for infective endocarditis due to the turbulent pattern of blood flow through these intracardiac channels [5, 6]. Therefore, PVL presence may influence the patient’s quality of life and, in some cases, treatment is life-saving.
Nowadays interventional procedures are being used to close PVLs with percutaneous or transapical access, with the latter approach reserved for more challenging cases [7].
The surgical approach is associated with a higher mortality rate and is reserved for treatment of PVLs with valve dehiscence (rocking valves) or those that accompany active endocarditis [8, 9].
From a procedural point of view, planning and imaging are crucial steps that significantly impact clinical outcomes [10]. The most common modality used for diagnosis and peri-procedural management of patients with PVLs is two- and three-dimensional transesophageal echocardiography (2D-TEE and 3D-TEE) [11]. Prior to the intervention, it is used to identify both the anatomical location and number of PVLs as well as to grade severity of regurgitation in all patients [8]. During the procedure, TEE (especially 3D-TEE) is critical in assessment of PVL size and shape and accurate measurements are essential for selection of the appropriate closure device [12].
Another useful tool for PVL imaging is electrocardiography-gated cardiac computed tomography (CT) with three-dimensional or four-dimensional reconstruction. However, the benefits of high temporal and spatial resolution are limited by artifacts from dense structures or high heart rate, risks associated with the use of contrast and (especially in younger patients) radiation.
The challenges with TEE or CT-based visualization of PVLs are related to the type of prosthesis (biological or mechanical), position of the prosthesis and location of the PVL and are similar between the two imaging modalities. Despite advancements in imaging techniques, it may not be possible to predict the final result of intervention prior to closure device placement in the PVL channel. The main reason for this is the lack of ability to adequately determine the shape and position of the occluder in the PVL channel after deployment and the risk of interference between the occluder and surrounding structures. The latter is especially relevant in patients with mechanical valve prostheses.
An incomplete seal of the PVL channel worsens the patient’s prognosis and increases the risk of post-procedural hemolysis. Failing to identify subjects in whom these complications may occur can lead to procedural failure [3]. Unpredicted interactions of the occluder with surrounding structures may lead to serious consequences, including blockage of the mechanical prosthesis or high risk of erosion.
To overcome these limitations 3D printing (until now based mostly on CT scanning) was proposed for planning of structural interventions [13]. It provides the unique possibility to visualize interactions between prosthetic discs, surrounding tissues and occluders used for PVL closure.
As the use of real-time 3D color Doppler imaging enables exact localization of the PVL it seems reasonable to use TEE data for 3D printed models. There are reports of using TEE-based 3D printings for cardiac interventional procedures [14, 15]. However, the preparation of 3D models from echocardiographic data is more challenging in comparison to CT due to lack of compatibility between echocardiographic (non-Cartesian) DICOM data and software used for stereolithography model preparation [16–19].
The feasibility of TEE-based 3D printing for planning and implanting PVL occluders is still unknown.
Aim
We aimed to test whether TEE-based 3D printed models could allow for the appropriate choice of an occluder for transcatheter PVL closure.
Material and methods
Study design
The data of all patients who underwent percutaneous mitral PVL closure around mitral valve prosthesis in the last year were reviewed. Eight consecutive cases with satisfactory quality of pre-procedural 3D-TEE recordings were chosen for the study. Based on TEE data, 3D models of each valve prosthesis and surrounding structures, including the PVL channel, were printed. In the next step, an investigator blinded to the results of transcatheter PVL closures tested different occluders on the 3D model following the rules used during the real procedures. Two types of closure devices were used: Paravalvular Leak Device (PLD; Occlutech) and Amplatzer Valvular Plug III (AVP III; Abbott), both of which are commonly used in our center during percutaneous PVL closure procedures. For larger PVLs with a short channel (< 5 mm) the Occlutech PLD was the first choice, whereas the tortuous course of the channel or length > 5 mm were indications for AVP III. In the case of larger PVLs, when a single device was not likely to completely close the leak, multiple plugs (up to 3 devices) were allowed [11].
In addition, results were correlated with actual occluders implanted in vivo.
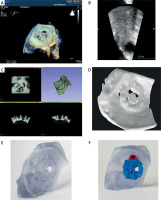
This study was performed according to the principles of the Declaration of Helsinki and did not require the consent of an Ethics Committee. All steps from image acquisition to printing are shown in Figure 1.
3D-TEE image acquisition
3D-TEE imaging was performed with the EPIQ7C ultrasound system (Philips Medical Systems, Andover, MA) with a fully sampled matrix transducer (X7-2t) according to a local protocol. All examinations were performed by 2 operators in accordance with the European Association of Cardiovascular Imaging (EACVI) recommendations under conscious sedation with diazepam administered intravenously [20].
Image transfer and segmentation
Images were transferred to the workstation and uploaded to a QLab station (Philips Medical Systems, Andover, MA, ver 3.8.5). Quality of images was assessed and recordings with satisfactory quality were identified. Selected non-color 3D volumes were exported in Cartesian DICOM (3DDCM) format. Next, the Cartesian DICOM files were uploaded into Slicer (Freeware software, v. 5.2.2 r31382/fb46bd1) [13], which is a free, open-source software package used for imaging research. To convert Cartesian DICOM to standard DICOM, the Philips DICOM Patcher module in Slicer was used (this step requires installation of the SlicerHeart and Sequences extensions). Semi-automatic segmentation of volumetric data was performed using thresholding with subsequent manual correction. Diastolic frames were selected for the best PVL channel visualization.
3D printing
All .stl files were imported into the 3D printer software. Afterwards the models were printed to actual size with the Polyjet printer (Stratasys, Commerce Way, Eden Prairie, MN, Objet 30 v.3). Rigid printing (IORA Model) and support (IORA Support) materials (Isquared) were used. Printing accuracy of the Stratasys Objet 30 printer, provided by the manufacturer, for models printed with rigid materials is based on the actual size of the model and is reported as maximal size deviation from the original size of the model – for model dimensions under 100 mm maximal deviation is ±100 µm [17].
Results
We retrospectively evaluated pre-procedural 3D echo scans of patients with mitral PVLs who underwent transcatheter closure. From this population, 8 patients’ data were selected for assessment and printing. Characteristics of printed PVLs are presented in Table I.
Table I
Types of valves, position of PVLs and model preparation time
The occluder implanted during the actual procedure corresponded well to the 1 suggested in 7 patients (87.5% of the study group). In 1 case, during the invasive procedure, the PLD occluder was initially selected, but due to significant deformation it was replaced with two AVPIII occluders, which aligned well with the benchmark study.
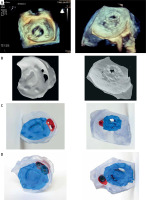
Table II presents procedural and pre-procedural data. Figure 2 shows corresponding 3D-TEE images (2A), Slicer models (2B), 3D-printed models (2C) and 3D-printed models with occluders (2D). Figure 3 illustrates 3D-printed models of PVLs, matched with different types of tested occluders.
Table II
Comparison of the benchmark testing and real-life data
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Type of implanted occluder | PLD-W | PLD-W | AVP III | PLD-W | PLD-W | PLD-W | AVP III | PLDW |
| Size of implanted occluder | 12x5 | 12x5 | 8x4 and 6x3** | 6x3 | 4x2 | 12x5 | 10x5 | 6x3 |
| Number of implanted occluders | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 |
| Occluder(s) proposed in benchmark testing | PLD-W12 × 5 or AVP III 14 × 5 or 3 × AVPIII 6 × 3 | PLD-W 12 × 5 | 2 × AVPIII 8 × 4 | AVP III 8 × 4 | PLD-W 4 × 2 or AVP III 6 × 3 | PLD-W 12 × 5 | AVP III 10 × 5 or 3 × AVPIII 6 × 3 | PLD-W 6 × 3 |
| Residual leak in vivo | No | No | Yes | Yes | No | No | No | No |
| Interaction with valve in vivo | No | No | No | No | No | No | No | No |
| One year survival after implantation | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
Discussion
In our study, we confirmed the feasibility of using 3D-printed mitral valve models to simulate PVL closure procedures, relying on data obtained from 3D-TEE imaging. Up until now, the literature has primarily focused on discussing the potential of CT images for 3D model preparation and transcatheter PVL closure [15, 21–24]. Our study adds to the existing knowledge by showing the practical application and benefits of 3D-printed mitral valve models in simulating PVL closure procedures, providing valuable insights for further advancements in this field.
The wide variety of occluders used for PVL closure posed a challenge in including the entire spectrum within the scope of this study. Therefore, two types of occluders, most commonly used in our center, were employed. This approach is consistent with common practice and, as demonstrated in the article by Hascoët et al., these two occluders (AVPIII – 56%, PLD – 14%) are also the most frequently utilized ones [25].
In our series of patients the empirical measurements corresponded well with sizes of implanted devices, but the study is only a demonstration of technical feasibility. The choice of a strategy is based on many variables and is beyond the scope of this article. One of the significant limitations is the small number of patients included in the study.
The PVL 3D model preparation is usually a time-consuming and complex process, due to the necessity of data re-formatting, manual segmentation of the images, the printing process and postprocessing [17]. Automation of these processes through dedicated software would significantly increase the potential usefulness of this method. In the literature there are examples where more sophisticated software is applied to prepare 3D models (Mimics 19.0, Materialise, Leuven, Belgium), which significantly reduces the model preparation time [26–28]. Unfortunately, license cost precludes everyday use of such software.
Another limitation of the study may be the type of the material used for printing. We decided to use rigid printing materials as they properly depict not only the location, size and complexity of the PVL, but also accurately represent the mechanical properties of the prosthetic valve and correspond well to the calcified structure of the aortic bulb. Rigid materials also properly depict the relationship between the prosthesis and surrounding tissues, but may unfortunately fail to accurately represent interactions between compliant tissues, as may occur in the case of mitral PVLs. Therefore some reports advise the use of multi-material 3D printing [29], and this may indicate the direction for further research.
Nowadays diverse models of virtual implantation of different intracardiac occluders are suggested. They allow one to partially predict the efficacy of occluder implantation and its interaction with surrounding tissues (FEops), but currently there is no virtual modeling available for assessing the performance of an occluder significantly deformed by a PVL channel [30].
Conclusions
Imaging data acquired during 3D-TEE can be used to prepare accurate 3D printed models of PVL.
3D-printed models of mitral PVLs allow the operator to simulate interactions with the defect before the procedure. It facilitates detection of interactions between the occluder, valvular prosthesis and surrounding tissues. Additionally, the model offers valuable insights to guide the operator in selecting the optimal type and size of the occluder.
The data processing methods used in the study are very time-consuming, and only automation of this process could increase the likelihood of practical application of TEE-based 3D models in everyday practice.
This is a hypothesis-generating study, and to draw stronger conclusions, a prospective study is necessary, followed by a study utilizing 3D modeling to guide the operator towards the optimal occluder selection.