Purpose
Hepatocellular carcinoma (HCC) is diagnosed when the disease has progressed to mid- or late-stage cancer due to lack of early symptoms [1]. Approximately 44.0-62.2% of patients exhibit macroscopic portal vein tumor thrombus (PVTT) when initially diagnosed [2]. The presence of PVTT can result in severe adverse effect on hepatic blood supply, leading to decreased liver function and poor patient’s prognosis. Median survival time of patients with HCC without treatment affected by PVTT is reduced to 2.5-4.0 months, as compared to 10-24 months in those without this comorbidity [3, 4].
At present, however, the optimal means of treating HCC complicated by PVTT remains uncertain. The Barcelona Clinic Liver Cancer (BCLC) group recommends that sorafenib is the therapeutic agent of choice for patients with advanced HCC, irrespective of PVTT status. However, sorafenib hepatocellular carcinoma assessment randomized protocol with BCLC recommendations, suggests that vascular invasion is present in only 38.4% of patients with HCC [5, 6]. As such, only sorafenib may not necessarily improve survival in patients with HCC and PVTT. In addition, studies on Asian populations with vascular invasion or extrahepatic metastasis have suggested that treatment with sorafenib was associated with poor survival time (median, 6.5 months) [6]. Transcatheter arterial chemoembolization (TACE) with sorafenib (TACE-S) exhibited therapeutic synergy when employed in treatment of HCC patients with PVTT, and provided good patency for the main portal vein or sufficient collateral circulation [7]. This combination is associated with some increase in patients’ survival. However, prolongation of survival in patients with main portal vein invasion is limited [6, 8]. Hence, a superior combination strategy is needed.
The use of a portal vein stent (PVS) to reduce portal vein occlusion has been explored in the context of PVTT [9]. Its’ combination with iodine-125 (125I) seed brachytherapy offers a potentially optimal means of improving PVTT management in patients with HCC [10]. PVS insertion and endovascular 125I seed-strip implantation combination with TACE-S had a potential therapeutic effect on HCC PVTT patients, but so far, that has not been fully elucidated [11]. In the present study, a retrospective analysis was performed to assess the efficacy of PVS insertion and endovascular 125I seed-strip implantation, followed by TACE-S (PVS-125I TACE-S) as well as the factors associated with patients’ outcomes.
Material and methods
This was a retrospective analysis of 53 patients with HCC with type II or type III PVTT, from May 2014 to July 2018, who underwent PVS-125I TACE-S or TACE-S. This study received approval from the ethics committee of our institution (No. LDYYLL2019-204), and all participants provided written informed consents.
In all patients, HCC diagnosis was confirmed either via histology or by a combination of two imaging approaches and elevated alpha-fetoprotein, as per the American Association for the study of liver diseases guidelines [11]. PVTT diagnosis was confirmed following an identification of low-attenuation intraluminal mass, partially or completely occluding the portal vein, or a filling defect in the portal vein, which were observed from three-phase dynamic computed tomography (CT) or magnetic resonance imaging (MRI) [12]. Patients were stratified into types I-IV PVTT according to Cheng’s PVTT classification system as follows: type I – tumor thrombi located at the segmental branches of the portal vein or above; type II – tumor thrombi extending and involving the right or left portal vein, type III – the main portal vein trunk involved; type IV – tumor thrombus extending to the main portal vein and the superior mesenteric vein [13].
Study inclusion criteria were as follows: 1. Diagnosis with HCC and either type II or type III PVTT; 2. No previous local treatment of PVTT lesions; 3. The Eastern Cooperative Oncology Group performance status of 0-2; 4. Child-Pugh class A (score 5 or 6) or B (score 7-9). Study exclusion criteria were as follows: 1. Complete portal vein occlusion with a lack of collateral circulation; 2. Bleeding of the esophagus or gastric fundus; 3. Intractable coagulation disorders; 4. Macroscopic hepatic vein tumor thrombi or extrahepatic tumor metastasis; 5. Lack of baseline imaging results.
Treatment schedule, including TACE, sorafenib, TACE-S, PVS-125I TACE-S, and stereotactic body radiotherapy (SBRT) was conveyed in detail to all participants, and the advantages and disadvantages of all treatments were discussed with the patients, who themselves chose the treatment method independently. During the study period, 72 patients received the afore-mentioned treatment, 12 patients with TACE, 5 patients with sorafenib, and 2 patients who chose SBRT were excluded. Finally, 53 patients were included in this study, of these, 28 patients who decided upon the combination of PVS-125I TACE-S formed group A, while the remaining 25 patients who chose TACE-S alone formed group B.
Portal vein stent insertion and endovascular 125I seed-strip implantation
The required number (N) of 125I seeds (GMS Pharmaceutical Co., Ltd., Shanghai, China) was determined based upon the length (L) of the obstructed segment of the portal vein in millimeters, using the following formula: N = L/4.5 + 2 [10]. The estimated radiation doses were roughly 40-50 Gy, as determined at specific dose reference points based on calculations conducted by a computerized treatment planning system (FTT Technology Co., Ltd., Beijing, China). Iodine-125 seeds used in this study were 0.8 mm in diameter and 4.5 ±0.5 mm in length, with 25.9 MBq of radioactivity and 59.4-day half-life, primarily emitting 27.4 and 31.4 keV X-rays and 35.5 keV γ-rays. Given the local tissue half-value thickness of 17 mm, these seeds were associated with an initial dose rate of 0.07 Gy/h. Prior to implantation, seeds were loaded in a linear arrangement in a 4-Fr flexible stiffening cannula (Boston Scientific; Marlborough, MA, USA) to construct 125I seed-strip. With ultrasound guidance, a Neff percutaneous access set (Cook Medical; Bloomington, IN, USA) was then used to puncture the patent second-order branch of the portal vein, following which, both a vascular stent (Bard Peripheral Vascular Inc.; Tempe, AZ, USA) with 12-14 mm diameter and 60-100 mm length and 125I seed-strip were implanted in a sequence. After PVS insertion and endovascular 125I seed-strip implantation, a 3-3 spring coil (Cook Medical; Bloomington, IN, USA) was used for blocking the intra-hepatic puncture. Subsequently, subcutaneous low-molecular-weight heparin (Changshan Biochemical Pharmaceutical Co., Ltd.; Shijiazhuang, China) 4,100 IU was administered twice daily, for a 5-day period. Next, warfarin (Shanghai Sine Pharmaceutical Laboratories Co., Ltd.; Shanghai, China) was administered orally to achieve international normalized ratio of 2.0-2.5.
TACE procedure
In the group A patients, TACE was conducted 3-7 days after PVS insertion and endovascular 125I seed-strip implantation, whereas in the group B patients, this procedure was performed directly. A 5-Fr hepatic-curve catheter (Terumo Corporation; Tokyo, Japan) was placed into the celiac artery and then, hepatic arterial angiography and indirect portography were performed. A 2.7-Fr micro-catheter (Progreat™, Terumo Corporation; Tokyo, Japan) was placed into tumor-feeding arteries, and a 5-20 ml of lipiodol (Wh-Medical Apparatus and Instruments Co., Ltd.; Beijing, China) mixed with 50-75 mg/m2 doxorubicin hydrochloride (Hisun Pfizer Pharmaceutical Co., Ltd.; Shanghai, China) was injected into the arteries. If the entire 20-ml volume was administered without substantially impairing blood flow in vessels, polyvinyl alcohol (PVA) particles (ALICON Pharmaceutical Science and Technology Co., Ltd.; Hangzhou, China) were used for vessel embolization, and administered until a limited slow flow was evident only. In patients with arterioportal shunts, initial embolization using 350-1,000 μm PVA particles was conducted prior to lipiodol/doxorubicin infusion to ensure shunt occlusion. After a month, the effects of TACE were assessed by contrast-enhanced abdominal CT/MRI, and a next treatment plan was determined.
Sorafenib
Sorafenib (Nexavar®, Bayer HealthCare; Leverkusen, Germany) is a small molecule that inhibits tumor cell proliferation and tumor angiogenesis to achieve therapeutic effect [14]. In this study, all 53 patients received sorafenib. The patients were administered 400 mg twice per day, for 3 to 7 days after TACE treatment, when liver function had stabilized. Sorafenib doses were reduced in many patients suffering from grade 1 or 2 adverse events (AEs), or with serum bilirubin > 34.2 μmol/l. Sorafenib treatment was temporarily halted in patients suffering from grade 3 AEs or hyperbilirubinemia (serum bilirubin > 51.3 μmol/l) events, and was resumed only if AE grades and serum bilirubin levels returned within the acceptable treatment ranges. The patients received sustained-release sorafenib tablets as long as possible, even when disease progression was noted, or until death.
Follow-up and treatment evaluation
Patients’ follow-up was conducted at 1 and 3 months after the procedure, and every 3 months thereafter. Follow-up assessment included a combination of physical examination, laboratory testing, and a contrast-enhanced abdominal CT or MRI. The Common Terminology Criteria for Adverse Events (CTCAE) v. 4.0 assessment was used to rate complications associated with the treatment [15]. Albumin-bilirubin (ALBI) scores were applied as an objective means of gauging liver function, with scores being calculated solely based upon albumin and bilirubin levels [ALBI score = log10 bilirubin (μmol/l) × 0.66) + (albumin (g/l) × –0.0852)] [16]. The modified response evaluation criteria in solid tumors (mRECIST) criteria for HCC were applied for tumor responses, with possible responses consisting of complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) (Figure 1). A disease control rate (DCR) after 6 months of treatment was calculated based on the following formula: (CR + PR + SD)/total cases × 100% [17]. Overall survival (OS) was the primary end-point defined as the time duration between start of treatment and either death or last follow-up.
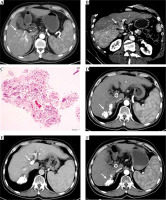
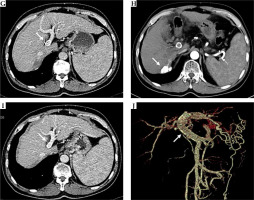
Fig. 1
A) Results from a 63-year-old male patient who had HCC with type III PVTT. Contrast-enhanced CT scan exhibiting a hepatic arterial phase hyper-attenuation lesion at segment 6. B) Segment 7 and PVTT extending to the main portal vein. C) Histopathological examination of the biopsy tissue sampling showed obvious cell atypia, different sizes and shapes, and deep nuclear staining. Some of the cells were acidophilic, with diffuse arrangement and necrotic tissue around, consistent with the morphological features of HCC. D) Hematoxylin and eosin stain, 100× results from the 3-month follow-up following the combination of PVS-125I TACE-S, lipiodol accumulation in the tumor. E) The observed satisfactory patency of the stent. F) Results from the 6-month follow-up, with the treated lesion decreased in size. G) The observed satisfactory patency of the stent. H) Results from the 12-month follow-up, the treated lesion continuously decreased in size. I) The observed satisfactory patency of the stent. J) Vascular reconstruction showing stent and 125I seed-strip correctly implanted in the portal vein without displacement
Statistical analyses
SPSS 21.0 (SPSS Inc.; Chicago, IL, USA) was used for all analyses. Quantitative results were expressed as means ± standard deviation, and t-test was applied to compare these values. Qualitative results were expressed as a number (%), and Pearson χ2 test or Fisher’s exact test were used to compare these results, whenever appropriate. Paired-samples t-test was used for comparison of ALBI scores in the patients of group A. Survival was analyzed via Kaplan-Meier approach and using log-rank tests. P < 0.05 was the significance threshold. Cox univariate and multivariate regression analyses were used to assess the relationship between specific factors and treatment outcomes.
Results
Baseline information
Baseline characteristics of the 53 patients are shown in Table 1, with no significant clinically relevant differences between groups. For the patients of group A, on average, 18.2 ±1.7 (range, 16-20) 125I seeds were implanted.
Table 1
Patients’ demographic and clinical characteristics
* Data obtained with Pearson χ2 test; # Data obtained with independent sample t-test; ECOG – Eastern Cooperative Oncology Group; HBV – hepatitis B virus; HCV – hepatitis C virus; PVTT – portal vein tumor thrombus; AFP – α-fetoprotein; TACE – transcatheter arterial chemoembolization; value of p < 0.05 was considered statistically significant difference
Complications and clinical outcomes
The most frequent AEs in the group A during PVS insertion and endovascular 125I seed-strip implantation were fever (3 patients, 10.7%), abdominal pain (20 patients, 71.4%), and a transient decrease in liver functionality (7 patients, 25%). Patients who experienced these AEs were recovered after conservative management. Furthermore, no serious procedure-related complications, such as abdominal hemorrhage, biloma, stent migration, or puncture site bleeding, were observed. Table 2 shows AEs occurring immediately during TACE-S, including nausea or vomiting, fever, and abdominal pain as short-term side effects in group A in 5 (15.5%), 3 (4.6%), and 7 (2.3%) patients, respectively, and in group B in 5 (15.5%), 3 (4.6%), and 7 (2.3%) cases, respectively. All of these AEs were resolved after symptomatic treatments. Long-term side effects, such as fatigue, diarrhea, hypertension, hand-foot syndrome, alopecia, pruritus, rash or desquamation, voice change, anorexia, and abscess occurred in group A in 9 (32.1%), 12 (42.9%), 10 (35.7%), 16 (57.1%), 2 (7.1%), 4 (14.3%), 3 (10.7%), 1 (3.6%), 4 (14.3%), and 1 (3.6%) patients, respectively, and in group B in 8 (32.0%), 10 (40.0%), 9 (36.0%), 13 (52.0%), 2 (8.0%), 5 (20.0%), 3 (12.0%), 0 (0%), 3 (12.0%), and 0 (0%) patients, respectively. Usually, all of these long-term side-effects began 1-2 weeks after the treatment and alleviated after sorafenib dose adjustment, short-term interruption, medications, or puncture drainage. TACE-S-associated AEs showed no significant difference between the two groups (p > 0.05). All patients had no procedure-related mortality.
Table 2
Transcatheter arterial chemoembolization-sorafenib (TACE-S)-associated adverse events (AEs) for the two groups (%)
Pre-operative ALBI scores were not significantly different between group A and group B (–2.57 ±0.42 vs. –2.61 ±0.38, p = 0.724), nor did these scores differ at 1 month post-operatively (–2.62 ±0.46 vs. –2.20 ±0.59, p = 0.666). However, these scores were significantly different at 3 (–2.17 ±0.59 vs. –1.69 ±0.48, p = 0.007) and 6 (–2.28 ±1.23 vs. –1.47 ±0.31, p = 0.044) months post-operatively (Table 3). DCR was 71.4% in group A and 44.0% in group B (p = 0.043) after 6 months of treatment.
Table 3
Pre- and post-operative albumin-bilirubin (ALBI) scores in the two treatment groups
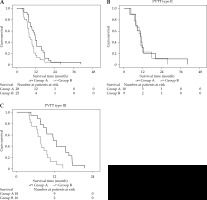
The median survival of group A was 11.4 ±0.7 months (range, 10.1-12.7), while that of group B was 7.7 ±0.8 months (range, 6.1-9.3 months) (p = 0.007). A stratified analysis demonstrated that the median survival in those with type II PVTT was 10.4 ±2.0 months (range, 6.5-14.4 months) and 10.7 ±1.7 months (range, 7.3-14.1 months) in group A and group B, respectively (p = 0.689), whereas in patients with type III PVTT, the survival time was 11.5 ±0.8 months (range, 10.0-12.9 months) and 7.5 ±0.9 months (range, 5.8-9.2 months), respectively (p = 0.002) (Figure 2).
Fig. 2
A) Kaplan-Meier overall survival curves from patients with HCC and PVTT treated via a combination of PVS-125I TACE-S (group A) or via TACE-S alone (group B). The entire study population (p = 0.007); B) Patients with type II PVTT (p = 0.689); C) Patients with type III PVTT (p = 0.002)
Univariate Cox model analyses suggested that tumor size and number were potentially associated with treatment outcomes, and as such, these were incorporated into a multivariate model. This analysis, in turn, determined that tumor size > 10 cm (p = 0.002) and multiple tumors (p = 0.022) were independent predictors of poor prognosis, whereas PVS-125I TACE-S was a predictor of favorable patient’s prognosis (p = 0.040) (Table 4).
Table 4
Univariate and multivariate analyses of variables associated with overall survival (OS) in hepatocellular carcinoma (HCC) patients suffering from portal vein tumor thrombus (PVTT)
Discussion
Nowadays, effective treatments for HCC with PVTT are limited and controversial. Liver resection can cure some patients with types I-III HCC [18]. However, post-operative long-term survival outcomes of these patients are poor due to high HCC recurrence rates, especially for early recurrence within 5 years of surgery [19]. Therefore, selection of the most appropriate treatment approach for these patients is critically important.
One approach to improve OS of patients with HCC/PVTT is radiofrequency ablation. However, this approach is associated with a relatively high-risk of injury of the portal vein. In addition, PVTT ablation can also cause bile vessel injury due to adjacent location of PVTT and bile vessels [4]. Also, transarterial radio-embolization has been used more commonly for patients with HCC and PVTT due to lower risk of hepatic ischemia and infarction, but it is not yet commercially available in certain regions, such as mainland China or Japan [20]. SBRT achieved a better therapeutic effect because the tumor could receive a higher dose of radiation directly. It was relatively safe, as the organ could also be protected from substantial radiation. However, tumor size was the main limiting factor with local control rates of 91% (< 5 cm tumors) and 74% (≥ 5 cm tumors) [21]. Some studies suggested that high-dose-rate brachytherapy (HDR-BRT), as an ablation technique using gamma irradiation of iridium-192 (192Ir) source, has successfully been used in HCC. In addition, HDR-BRT is not restricted to tumor size or tumors close to blood vessels or sensitive structures [22, 23]. However, research on whether HDR-BRT can benefit various types of PVTT is lacking.
In this study, PVS-125I TACE-S was employed. It was a well-tolerated and viable strategy for improving hepatic functionality and prolonging survival, compared with traditional TACE-S in patients with HCC with type III PVTT. The AEs related to PVS insertion and endovascular 125I seed-strip implantation, including fever, abdominal pain, and transient decrease in liver functionality, which were resolved after conservative managements. Decreased liver function is likely to result from injury to the bile duct upon puncture of the portal vein [24]. TACE-S-related AEs, such as nausea or vomiting, fever, and abdominal pain were relatively mild and were resolved after symptomatic treatments. Fatigue, diarrhea, hypertension, hand-foot syndrome, alopecia, pruritus, rash or desquamation, voice change, and anorexia were the most common sorafenib-related AEs, which could be managed by sorafenib dose adjustment, short-term interruption, and medications. Abscess rarely occurred and was resolved through puncture drainage. All AEs showed no significant differences between groups, which suggested that PVS-125I TACE-S was a well-tolerated intervention strategy.
In the present study, pre-operative ALBI scores were not significantly different between groups (p = 0.724), nor did these scores differ at 1 month post-operatively (p = 0.666). However, those scores were significantly different at 3 (p = 0.007) and 6 (p = 0.044) months post-operatively. Patients with HCC and PVTT had relatively poor basal liver function due to portal vein invasion, and liver function was aggravated after repeated TACE-S. It was postulated that this might be due to multiple reasons, including liver function damaged by chemotherapy and embolization that could cause damage to normal liver tissue, further aggravating the loss of the remaining liver function. Additionally, sorafenib itself has a potential to reduce portal blood flow, increasing the risk of liver failure [18, 25, 26]. However, in group A, PVS insertion and endovascular 125I seed-strip implantation were performed before TACE-S. PVS provided immediate restoration of the blood flow of obstructed portal vein, improving the hepatic blood supply. In addition, as 125I seed-strip was used for sustained intravascular brachytherapy, a suitable means of counteracting neointimal hyperplasia and improving stent patency duration is required [24]. The combined therapy can lead to long-term re-canalization of the portal vein, and hence, liver function is improved to varying degree.
Also, better DCR was observed in group A rather than in group B (71.4% vs. 44.0%) after 6 months of treatment. TACE was reported to block the flow through arteries, which supplied blood to the tumor, thus allowing for the control of tumor growth and PVTT progression, since the hepatic artery was the primary source of blood for tumor cells and thrombi [17]. Complete local necrosis was often difficult to achieve because this region had blood supplied by both the artery and portal vein, and it had the potential for collateral arterial development [27, 28]. TACE was also associated with elevated levels of vascular endothelial growth factor, resulting in a higher risk of local recurrence [27]. Sorafenib combination treatment reduced TACE-associated risks and improved outcomes. However, some reports suggested that sorafenib was less effective in those with Vp3/4 PVTT (< 10% of response rate) [6, 9]. In contrast, 125I seed implantation in the portal vein allowed for effective PVTT control owing to sustained low-dose X- and γ-ray release throughout the tumor area, damaging tumor cell DNA and disrupting proliferation [11, 29, 30].
In a sub-group analysis, a significant difference in OS was observed in group A only for patients with type III PVTT (11.5 vs. 7.5 months). This might be because the near-total portal vein blockage in patients with type III PVTT was linked to a rapid decrease in liver function [31]. The liver function could be aggravated by repeated TACE and long-term sorafenib application, as described earlier. This, in turn, required modifications or discontinuations of TACE-S treatment regimens, potentially constraining their therapeutic value [32, 33]. PVTT in the main portal vein can also increase portal vein pressure, causing fatal acute variceal bleeding [10]. The PVS insertion and endovascular 125I seed-strip implantation strategy improved portal vein obstruction, thereby reducing the risk of esophageal and gastric bleeding [34, 35]. In addition, 125I seed implantation improved local tumor control and delayed PVTT progression, and possibly reducing the risk of local and distant metastases [36]. No significant differences in OS were observed in patients with type II PVTT in the two treatment groups of the present study (10.4 vs. 10.7 months). This might be because PVTT involved only the first-order portal vein branch, with TACE-S achieving a better therapeutic effect [8].
The multivariate analyses showed that tumor size and multiple tumors were independent predictors of OS, and this was consistent with previous findings [37]. Additionally, the treatment strategy was an independent predictor. However, no positive results were obtained for PVTT typing, probably because of PVS-125I TACE-S treatment protocol, which could be more important for prolonged survival.
This study had certain limitations. First, the sample size was small, which undermined the conclusion. Second, this study was retrospective in nature. Despite efforts to control potential confounding factors, a future randomized controlled trial is needed to validate the results. Lastly, a skin surface dosimeter was not employed after 125I seed-strip implantation, making it impossible to determine the exact radiation doses administered to the patients in this study.


