Introduction
Mechanical thrombectomy (MT) along with intravenous thrombolysis (IVT) is a well-established class I A treatment for acute ischemic stroke with large vessel occlusion (LVO) [1–5] and supported by Polish, European and US guidelines [6–8]. In a meta-analysis of five pivotal MT trials, the number needed to treat (NNT) of 2.6 for functional independence at 90 days has left even the most optimistic enthusiasts of invasive stroke treatment aghast [9]. MT not only improves functional status in LVO stroke patients, but also reduces mortality and in conducive circumstances may lessen the cost burden on healthcare systems [10, 11]. While the MT Pilot Program in Poland is about to end soon, we still provide this breakthrough treatment to 4% of patients with ischemic stroke [12]. In many western countries, this number is significantly higher and even careful estimates suggest that MT-eligible LVO patients may constitute approximately 5–17% of ischemic strokes [13–18]. In spite of the continuous development of better and more efficient clot retrieval devices, obvious systemic obstacles to progress still exist. Much as was the case with acute myocardial infarction management in the post-thrombolytic era, there is now an urgent need for the establishment of a fully operational network of mechanical thrombectomy centers in Poland. In fact, the creation of a Polish primary Percutaneous Coronary Intervention (PCI) network, with angio suites established even in small centers closer to a patient, may be a case study for the development of interventional stroke therapy. In this article, the authors – cardiologists and neurologists, who are members of a multidisciplinary team – try to identify those areas where acute ischemic stroke treatment can be improved in Poland.
MT Pilot Program in Poland
The Mechanical Thrombectomy Pilot Program was started in 2019. After two years, the Presidium of the Supreme Medical Council in Poland called for immediate expansion of the present MT network to improve patient access to mechanical thrombectomy [19]. In its reply letter (available online), the Cerebrovascular Section of the Polish Society of Neurology declared it believed that 1 Comprehensive Stroke Center (CSC) per million inhabitants would suffice. With 20 Comprehensive Stroke Centers (CSC) active in the Pilot Program and 38 million inhabitants of Poland, we are far from this optimistic assumption, since this is half of what is expected (0.52 per 1 million). Considering this, at least 40 centers should be in operation in Poland right now. After 3 years of the Pilot MT Program, the number of ischemic stroke patients treated invasively is 4.0% [12] and falls short of what neurologists themselves consider reasonable (in the same statement of the Neurovascular Section of the Polish Society of Neurology of 2020, it was postulated to be between 5 and 10%). The position of the European Society of Cardiology Council on Stroke is that to fully and timely cover for contemporary stroke needs, at least 2 MT centers per million population are required [20]. For Poland, that would mean creating around 56 additional mechanical thrombectomy centers.
After the due completion of the Pilot Program in Poland, there are many burning questions to be addressed. First, what are the achievements and failures of this program? What are the main obstacles to further improvement in stroke care? What should the present network transform into when the MT Pilot Program ends? How many centers per 1 million inhabitants are truly required when a likely rapid influx of latecomers invisible in the Pilot Program (DAWN and DEFUSE-3 protocols) occurs? Does the network of 20 Comprehensive Stroke Centers (where many patients cannot arrive in time) really suffice or should we follow the example of other European countries and create a wider network of Thrombectomy-Capable Stroke Centers (TCSC)? How many new operators do we need and what formal training is required to provide for fast growing needs in the not too distant future? There is certainly a need for a clear and transparent audit system where data from all hospitals already involved in the MT program would be available to assess the quality of services. It should focus on both safety (MT failure, symptomatic intracranial hemorrhage – sICH and 30-day mortality rates) and efficacy (first-pass recanalization, mean NIHSS drop and 3-month mRS rates) outcomes. Data should be collected and presented on a regular basis and ways to improvement discussed. Should a given MT center’s performance diverge strikingly from the national average, retraining of the team ought to be mandatory. Is it possible to compare head-to-head results from different centers and regions to know which system of stroke care prevails?
To best serve Polish patients, we need answers to two key questions: what model of MT service network should Poland adopt, and consequently, what number of MT centers per region should be established? Do densely populated regions need the same number of MT capable centers per voivodship compared to rural areas? The right decision is crucial as it will shape the structure of the MT network for years to come. There are two viable alternatives: a centralized and a decentralized system.
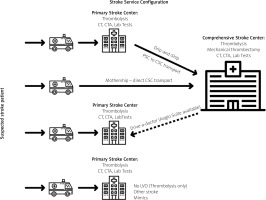
In a centralized model based on Comprehensive Stroke Centers, large central hospitals in big cities cover vast areas and transport MT candidates from multiple Primary Stroke Centers (PSC). Huge neurointerventional experience and accumulated manpower are the definite strengths of this system. Such high-volume centers will usually perform several hundred procedures per year and have great expertise in the field [21].
A decentralized system would be based on 3 to 5 Thrombectomy-Capable Stroke Centers per region (voivodship) with crucial MT services brought closer to a patient’s location, thus mitigating the weaknesses of a centralized system with long transport time. Locally available operators after proper mechanical thrombectomy training would provide treatment for acute ischemic stroke with LVO [16, 22–26]. This model would not only efficiently shorten the crucial time to reperfusion but also prevent undesired patient accumulation observed in sparse CSCs today.
In Poland, the centralized system has been in place for 3 years of the Pilot MT Program. The 7th online Symposium on Ischemic Stroke (December 2021) was a great opportunity for 17 teams of this program to assess its strengths and weaknesses [12]. Some clear problems of such a model emerged, the most important issues being: considerable delays in transportation, problems with proper LVO patient qualification, issues with information flow, problems with radiology services, cases of vanishing local PSCs, nursing staff shortages, lack of a comprehensive data analysis system or modeling tools to create the best region-specific patient management strategy.
Transportation delays
In Poland, where drip-and-ship (DS) is the dominant model, delays are not only the result of long distances between PSCs and CSCs, but also due to the common problem of ambulance availability. Establishing a ‘standby ambulance’ service at every hospital seems to be the best ad hoc remedy, yet is unlikely to be promptly employed in Poland for economic reasons. Rapid transportation is of utmost importance in acute ischemic stroke, where 1.9 million neurons die every minute [27]. Not only is treatment delay associated with poorer functional outcomes [28], but also long distance transport seriously affects survival [29]. A more comprehensive and future-proof solution to the problem would be to build a network of Thrombectomy-Capable Stroke Centers so that the transport of patients from anywhere in the region does not exceed 1 h. This appears to be the only solution that truly addresses the problem since even in very well organized centralized systems, inter-hospital transfers prolong onset-to-arrival time by over 140 min [30].
Better prehospital LVO stroke detection
Since clinical outcomes for both IVT and MT are highly time-dependent [30, 31], it is crucial to promptly separate potential LVO patients to ensure their timely access to endovascular treatment (EVT). Given that mobile computed tomography (CT) ambulances are not widely available, multiple clinical assessment LVO scales have been developed [32]. These scales differ with respect to sensitivity and specificity, with a positive predictive value of around 50%, and with the highest predictive power to detect LVO observed in the VAN (Vision, Aphasia and Neglect), LAMS (Los Angeles Motor Scale), NIHSS (The National Institutes of Health Stroke Scale) and RACE (Rapid Arterial OCclusion Evaluation) scales [7]. Despite the lack of evidence to support the use of clinical stroke scales in routine pre-hospital triage [7], it seems reasonable to select and introduce one of them into ambulance crew training nationwide to facilitate the triage process and decision-making. Creating additional MT capable centers would reduce the risk of inappropriately classified patient accumulation in scarce CSCs.
Information flow
In the situation of acute brain ischemia, time plays a crucial role and information flow must be addressed. Establishing reliable liaisons between ambulance services and stroke centers with a prompt pre-notification process is the cornerstone of an efficient network [33]. A good system should include established alarm telephone numbers and decision paths for both ambulance services and acute stroke neurology departments. Smartphone-based telephone and tele-transmission consultations from an ambulance may help to make a timely and correct decision where to refer the patient first [34]. The ideal solution would be to create a single network of internet communication channels between PSC and MT centers in order to quickly transfer data and make swift decisions (e.g., e-stroke mobile application). Research is currently underway on modern technologies such as telemedicine, biomarkers and infrared screening devices to improve pre-hospital differentiation between ischemic stroke and stroke mimics [35]. Both centralized and decentralized systems will need to address all these issues.
Radiology services
Efficient stroke treatment cannot exist without good quality radiology services. In the MT Pilot Program, many centers reported far from ideal computed tomography/computed tomography angiography (CT/CTA) services at PSCs [12]. Delays or a lack of precise radiological assessment (particularly where teleradiology was involved) was a matter of serious concern. It has been postulated that standardized protocols for CT/CTA should be established in each stroke center to facilitate swift decision-making by consulting MT teams. The planned introduction of the POLCARD-financed advanced CTA postprocessing system called Brainomix may further improve and speed up the process. In a decentralized system, some of these issues would be bypassed, since many more patients would go directly to a Thrombectomy-Capable Stroke Center, where consultant radiologists would be accustomed to operating along MT operators and there would be fewer problems with communication.
Local stroke units crisis
It has been reported that, due to shortages of personnel, local stroke units started to disappear in some areas [12]. It would require a fundamental change in reimbursement philosophy, backed by patient lobbying groups, but it seems obvious that creating more and better equipped Thrombectomy-Capable Stroke Centers would also be a partial remedy to this unfortunate situation. TCSC would provide more comprehensive services to stroke patients providing both IVT and MT locally.
Nursing staff
We have been facing unprecedented service disruptions due to personnel shortages and a lack of proper funding [12]. Acute stroke patients require very close monitoring and it must be emphasized that nursing care in stroke units involves different staffing standards and policy than in other departments. Government agencies responsible for reimbursement must be made aware of the specific situation of stroke unit patients. Unless this issue is resolved, we are about to witness further loss of key personnel and growing instability of the system.
Treatment model selection
Today there are three main treatment models of LVO-suspected patients: mothership (MS), drip-and-ship (DS) and drive-a-doctor/drip-and-drive (DD). So far there is no strong evidence of a clear advantage of either of the transportation models (DS vs. MS) and they should be applied according to local organizational logistics and individual characteristics of the patient while other models are still in the early phase of assessment. Presently the MS model is favored in highly populated areas where patient transportation to the CSC is below 30–45 min and DS is preferred above this time limit [7].
Mothership (MS)
In the MS model, stroke patients bypass local PSC and are brought to a central institution which itself selects those who should be treated with MT. In this model, seldom preferred by teams audited during the 7th Symposium on Ischemic Stroke (December 2021) [12], there are severe logistical problems with considerable numbers of stroke patients, both MT and non-MT treated, accumulated in one hospital. Their relocation to local hospitals proves difficult and thus this model is seldom accepted. The MS model may be effective in an extensive hospital network with many MT centers accepting shared quotas of patients. The establishment of rehabilitation services in these units would allow for the quick relocation of many patients.
Drip-and-ship (DS)
The DS model is constructed to bring patients to the nearest stroke unit (PSC) where fast clinical and radiological assessments are made. The potential beneficiaries of this approach include patients from remote locations who are promptly diagnosed and provided with treatment. If a patient qualifies for mechanical thrombectomy, transfer to an MT stroke center is then arranged with an active drug (i.e., thrombolytic agent) on board. This model is widely accepted in centralized systems since MT teams are consulted after the diagnostic process and the first selection of patients takes place on site, thus eliminating transport of MT-ineligible patients. This system gives some control of the patient flow to MT centers but significantly prolongs time to mechanical intervention when LVO is detected. In the Catalonia stroke network based RACECAT trial (unpublished; presented at ESOC in 2020 by Marc Ribo and Natalia Pérez de la Ossa) results for both DS and MS models were comparable, yet it must be emphasized that the logistics and time measures presented in this study were exceptionally good and thus unlikely to be replicated. The decentralized model offers shorter transportation times to local TCSC and makes benefits of the DS approach less obvious.
Drive-a-doctor/drip-and-drive (DD)
This model is the least popular. It has been proposed for two reasons. Firstly, to avoid lengthy transportation delays, a patient is diagnosed and treated on site with an operator arriving at the patient’s location. The other reason was for MT to be provided by experienced operators ‘hired’ from high-volume centers. The weaknesses of this solution are more than obvious. There is not enough manpower to sit and wait ‘on call’ for an MT alert. It is economically difficult to support the accumulation and storage of necessary equipment in centers not using it on a daily basis. Investment in costly angiography equipment that might be underutilized must be taken into account. Another disadvantage of this model is the lack of the visiting neurointerventionalist’s familiarity with a local angio suite, as well as possible inexperience of supporting staff infrequently performing such procedures. This model may be best suited to very remote hospitals with poor transportation services, yet adequately equipped [36]. The pros and cons of the models are presented in Table I.
New paradigm in operator training
Unfortunately, training MT operators is still a divisive and political issue. There is no doubt that the shortage of MT-trained operators is a great challenge [20, 26]. The growing demand for timely and effective stroke treatment in Poland was a stimulus for the extension of the spectrum of MT operators well beyond the few neuroradiologists by the Minister of Health [37]. With MT available only in scarce neuroradiology-based CSCs, it may be considered the main reason for inadequate access of patients to proper treatment [38]. There is growing understanding, also strongly supported by the World Federation for Interventional Stroke Treatment (WIST), that interventional cardiologists with previous peripheral and carotid expertise will be quick to obtain MT skills and therefore their training may be much shorter [38, 39]. For experienced interventionalists, training at neuroradiology centers of excellence along with structured supervised silicone and cadaveric model exercise may be the best and shortest way to achieve necessary MT competence without compromising patient safety [40].
The scale of the problem
The Polish National Health Fund (NFZ) monitors closely numbers of patients with acute stroke and present quality of care delivered with regard to thrombolysis and mechanical thrombectomy. We know well the number and costs of the provided therapies from the online NFZ databases. What we are completely unaware of is the cost associated with delayed or abandoned treatments resulting from untimely access to treatment. We realize that there are many patients who have been failed by the present system, but since they are not reported, we cannot see the scale of the need that is unmet [39, 41, 42]. Given the number of mechanical thrombectomy procedures performed in other healthcare systems in the world, we can only estimate that we provide good services to less than half of the eligible patients [13–17]. In 2020, approximately 90,000 patients diagnosed with acute stroke were hospitalized in Poland, with roughly 75,000 identified as ischemic. There were 2320 MT procedures performed within 12 months from November 2019, which makes around 3% of all ischemic strokes (online NFZ data for 2019 and 2020). In the UK, with a similar number of 95,000 stroke patients hospitalized yearly, 83,000 were diagnosed to be ischemic. Among those, 33,000 cases were diagnosed to be LVO strokes, of which 11,580 were thrombectomy-eligible (the sum of patients presenting with LVO within 6 h from onset and those presenting later with favorable perfusion criteria), which makes 13% of all ischemic strokes [43]. Germany with its staggering 150 MT-center-strong network treats 7.2% of ischemic strokes invasively [13], and the Czech Republic even more (7.5–8.1%) [16]. France, with almost twice as many MT centers at its disposal (2018) as Poland today, decided to implement considerable changes to improve access to endovascular treatment. Two groundbreaking postulates emerged, namely training operators outside the field of neurointervention to perform MT and creating additional, smaller and closer to the patient Thrombectomy-Capable Stroke Centers [44]. The newest European Stroke Organization (ESO) Guidelines on intravenous thrombolysis for acute ischemic stroke (2021) recommend extending the therapeutic time window for intravenous recombinant tissue plasminogen activator (IV rtPA) and MT to 4.5–9 h with CT/magnetic resonance imaging core/perfusion mismatch, so the expected number of MT-eligible patients may increase even more in future [45]. Acute ischemic stroke patient pathways in different transportation models are presented in Figure 1.
What needs to be done
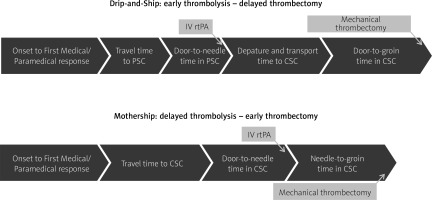
The detrimental effect of time passage in the acute ischemic stroke situation was clearly shown in the MR CLEAN trial, where each hour of delay resulted in a 7% drop in probability of a good clinical outcome [1]. Patients revascularized early, within 2 h of symptom onset, had a 33% absolute difference in good outcome compared to the control. This difference plummeted to 6.5% after 6 h of symptoms [46]. The selection of the best model of a stroke network and transfer system for Poland is urgent. There is no European Stroke Organisation (ESO) recommendation as to which of the two dominant transfer models (MS or DS) of LVO-suspected stroke patients should be favored; thus the choice ought to be made based on patient characteristics and regional service organization [7]. Comparison of time intervals in two dominant transportation models (MS and DS) is presented in Figure 2. The MS model may be preferred in urban areas where transport to the MT center is less than 30–45 min, while patients beyond this time limit may benefit more from DS [7]. Observational studies suggest that for suspected LVO stroke patients the MS model offers better functional outcomes [47–53]. The mothership model, compared with DS, offers shorter transfer times, 275 vs. 179.5 min, respectively [31]. Associated transfer delays result not only in worse clinical outcomes [54], but in a recent large study increased mortality as well [29]. In the ETIS registry, the mothership paradigm outperformed the drip-and-ship model with respect to 90-day functional independence (modified Rankin Score – mRS 0-2) 60% vs. 52%, respectively, as well as for excellent outcome (mRS 0-1), which was 9.6% higher in the MS model [55]. This analysis also assessed the clinical impact of both (MS and DS) models with respect to the PSC-CSC distance as well as the time interval between imaging and groin puncture. The MS paradigm was better for patients transported over longer distances (> 12.5 miles from PSC to CSC), or when the time from CT to puncture exceeded 140 min. In this analysis, despite the relatively short mean time of inter-hospital transport (43.5 min), no advantage of the drip-and-ship model was observed for patients localized further from CSC. The authors note that simple switching from DS to MS mode for LVO-suspected patients would not improve outcomes for all, since those who live in remote rural areas will always have longer travel times [55]. In another study, direct transfer to MT centers (MS) showed that thrombolysis would be delayed by only 12 min, while thrombectomy could be performed 91 min sooner compared to DS [48]. Researchers suggest that LVO-suspected patients should be treated by the MS model if the door-in-door-out (DIDO) time in PSC exceeds 40 min [56], or time from imaging to groin puncture exceeds 90 min [57]. In a recent meta-analysis of 7824 patients, the MS and DS models were compared [58]. There was no significant difference in onset-to-thrombolysis time, but in the MS model, the mean stroke onset-to-puncture time was significantly shorter, functional outcome was better, and the risk of symptomatic ICH was lower. The comparison of the DS and MS models has also been tested in the Polish healthcare environment [59]. Again, MS vs. DS comparison resulted in, as expected, significantly shorter times from onset-to-groin puncture (85-minute difference) and CT-to-groin puncture (84-minute difference), but there was no statistically significant improvement in functional outcomes (mRS 0-2) for direct transfer. Still, excellent functional outcome (mRS 0-1 at 90 days) was superior for the MS model compared to DS (33 vs. 23%, respectively). Karlinski in his commentary on this study concluded that even modest benefits observed in the MS model in the Polish healthcare system pointed to a potential advantage of this paradigm, should the MT system be better organized and reimbursed [60]. Schlemm used mathematical modeling to calculate additional-delay-to-thrombolysis thresholds associated with the greatest reduction in disability-adjusted life years and concluded that patients suspected of LVO ischemic stroke should be treated with the MS paradigm if additional delay to thrombolysis was < 30 min in urban and < 50 min in rural settings [61]. The notion that the MS paradigm trumps DS was challenged by the surprising results of a recent randomized controlled trial (RACECAT) in an exceptionally well-organized stroke network in Catalonia (unpublished). Good outcome defined as mRS 0-2, as well as mortality at 90 days, was similar in DS and MS groups. However, there is a significant caveat to this study: these results may be hard to replicate, since in this particularly well-organized stroke network, crucial time measures were, unlike anywhere else in the world, comparable in MS and DS models.
Figure 2
Comparison of time intervals in two dominant transportation models
PSC – primary stroke center, CSC – comprehensive stroke center, DS – drip-and-ship, MS – mothership.
Drip-and-ship is the preferred model in Polish reality today [12]. A glance at the location of the MT Pilot Program CSC centers on a map of Poland reveals the reason: there are many regions with long travel distances.
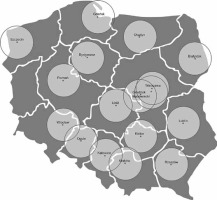
With only 20 CSCs providing MT, many towns lie outside a 70-kilometer circle to the nearest MT stroke center (e.g., Suwałki, Płock, Konin, Tomaszów Lubelski, Sandomierz, Jelenia Góra, Ostrów Wielkopolski, Piła, Koszalin, Słupsk, Ostrołęka, Mława, Giżycko, Sierpc, Wałcz, Szczecinek, Gorzów Wielkopolski). It may be estimated that ambulance transfer from these places to the nearest MT centers takes at least 2 h. Similar findings were reported in the HERMES meta-analysis, but it is also the real life experience of well-developed MT networks [30, 62]. Helicopter transport may be considered an option but in reality this is often unavailable on time due to other emergencies it constantly covers. Reports from Western Europe indicate that barely 4% of patients with acute stroke are transported this way [63]. It needs to be emphasized that transfer time is not transport time. The latter is around 36% of the transfer time, which also includes patient-associated delay, time to ambulance arrival on scene, on-site assessment time, telephone contact by ambulance crew with Stroke Center, and all delays associated with pre-hospital measures taken [64, 65]. When 50-kilometer circles are applied to 17 main regions on a map of Poland (Figure 3), most voivodships will accommodate at least 3 such circles. This rough estimate by no means indicates that the transport time within the circle would be limited to 1 h, since geography and road congestion are to be considered. Yet, even such a crude assessment gives us some information about transport difficulties within the present MT network.
Figure 3
‘No country for old men with stroke’ – map of Poland’s presently active 20 Comprehensive Stroke Centers (CSC), surrounded by 50-km radius areas with around 60% of country’s area potentially beyond fast access to mechanical thrombectomy (MT) services
There are sophisticated modeling programs (e.g. Destine Health) which help construct the most suitable transport models for stroke patients for specific regions and geography [66, 67]. They take into account geographical conditions, the efficiency of the local hospital system, and the best transportation system available on site. These algorithms include as specific data as different LVO screening stroke scales, crucial in-hospital and inter-hospital time intervals such as ‘door-to-needle’, ‘door-in-door-out’, ‘door-to-groin puncture’ and even take into account the proportion of patients being treated with IVT and MT in the region of interest. The modeling programs provide their user with an interactive map which helps predict the likelihood of a good outcome with different sets of transport protocols. This software has been successfully introduced in several different countries (e.g., USA, Japan, UK, Sweden and Germany) and may be useful when creating new or planning how to reorganize the existing stroke networks [https://destinehealth.com/].
Most centers taking part in the Polish Pilot MT Program were active participants of the recent 7th Symposium on Ischemic Stroke [12] and had the opportunity to share their experiences and name problems. So far, the DS model is a default in Poland, and for obvious reasons: transfer times between PSCs and CSCs are long. It seems impossible to improve patients’ timely access to treatment in either the MS or DS models without further expansion of the MT network. We believe that the discussion which model of treatment, DS or MS, would be better for Poland in 2022, is a substitute one. Recent research suggests that in most instances, in LVO-suspected patients, the MS model should be preferred since it offers a much better chance of a good clinical outcome. With the present limited network of MT Comprehensive Stroke Centers in Poland, we are highly unlikely to improve. In fact, we do need to considerably increase the number of Thrombectomy-Capable Stroke Centers at the cost of Primary Stroke Centers and bring services closer to the patient [47, 68–70]. With approximately 75,000 ischemic strokes diagnosed in Poland, we would have to perform 5,250–7,500 interventions yearly to achieve 7–10% of strokes treated by MT. If we managed to extend the MT network to 3–5 per voivodship, the number of centers would rise to 50–85. With 50 active MT centers (CSC and TCSC), approximately 105–150 thrombectomies per center would be performed yearly, not far from the currently observed mean value of 116 MT per center. This estimated case volume would put them in the high-volume range according to the Mission Thrombectomy 2020+ Global Executive Committee report prepared in cooperation with the Society of Vascular and Interventional Neurology (available online), established operator/center volumes recommendations [69], and a recent nationwide analysis in the US [21] with clear benefits of preserved patient safety, treatment burden more equally spread and improved outcomes. Considering the present shortage of operators and the time required for necessary MT training, it seems essential for specialists from various fields of medicine to get involved [16, 24, 41, 42].
Summary
Data from developed countries suggest that among ischemic stroke patients, 5–17% may be MT-eligible. In the centralized MT Pilot Program in Poland with 4.0% (2021) of ischemic strokes treated by mechanical means, there is an obvious medical need for improvement. So far, there is no clear answer as to which organizational model of transport in ischemic stroke patients is better (DS vs. MS), but neither will be efficient in a network with too few MT centers. Multiple observational studies and meta-analyses suggest that direct transfer of patients to MT centers – the mothership model – is of greatest benefit to patients. We strongly believe that whichever model is going to be locally applied, a decentralized and expanded system, staffed by operators from different backgrounds, with 3-5 Thrombectomy-Capable Stroke Centers per region, is what is required to cover the present MT needs in Poland. Crucial services should be brought closer to the patient, thus shortening times to revascularization and improving outcomes. Sophisticated modeling tools may help to construct the best paradigms for stroke management in a given region.
In this article, we have tried to analyze the strengths and weaknesses of the present MT stroke network in Poland. So far, discussions about the stroke care system have taken place within the safety of closed circles; hence the atmosphere was not very conducive to working out the project of the best system together. If this article may act as an invitation to an open discussion between various specialties on how to build a modern and effective network of ischemic stroke service, based on interdisciplinary teams, that would provide the best outcomes for patients in Poland.