Purpose
Prostate cancer is the second most common cause of cancer worldwide, and it is one of the main contributors to total disability-adjusted life years globally [1-3]. With a growing worldwide population, the number of person- years of life lost globally is estimated to increase from 3.5 million in 2020 to 7.5 million by the year 2040 [4]. Following primary radiotherapy, isolated intra-prostatic recurrence is of concern [5-8]. Recurrence rates vary based on initial prognostic factors, but an estimated 10% to 60% of prostate cancers may experience a biochemical recurrence [7, 9]. Treating intra-prostatic recurrence of prostate cancer after initial external beam radiotherapy (EBRT) poses a unique clinical challenge. Rosoff et al. found that salvage radical prostatectomy was associated with higher perioperative mortality and morbidity compared with primary radical prostatectomy. Therefore, salvage surgery is only a feasible option in selected patients due to its morbidity profile [10]. External radiation is often contraindicated as the bowel and bladder receive radiation doses close to tolerance limits during routine EBRT [10].
Salvage high-dose-rate brachytherapy (sHDR-BT) presents a potential solution for these challenging cases, as it allows for highly localized radiation dose to the prostate while minimizing the radiation to normal tissue. Another advantage is that it permits for simultaneous integrated boost that is delivered directly to the cancerous nodule with evidence supporting positive clinical outcomes, and manageable toxicity profile when combined with androgen deprivation therapy (ADT) [11]. Hence, sHDR-BT is a viable option for institutions that have advanced brachytherapy knowledge, technique, and expertise [12, 13]. Currently, there are no established guidelines on the use of sHDR-BT, and it is important to understand the potential adverse outcomes associated with this procedure. This study adds to the existing literature on the toxicity outcomes associated with it.
In this retrospective study, we aimed to report on the acute toxicity results from patients treated with sHDR-BT after initial radiotherapy treatments. There are some studies in the literature that report on the toxicity outcomes of sHDR-BT. Chitmanee et al. performed sHDR-BT among 50 patients with a one-time dose of 19 Gy [14]. The maximum acute gastrointestinal and genitourinary toxicities were grade 2, with 8% and 54% of patients experiencing them, respectively. There was no grade 3 or higher acute toxicities. Maenhout et al. investigated a cohort of 17 patients with one-time dose of 19 Gy, and reported the maximum acute genitourinary toxicity experienced by their cohort as grade 2 (11.8%) [15]. In a study by Slevin et al., 43 patients were treated with a dose of 19 Gy delivered in one fraction. They reported that the maximum gastrointestinal acute toxicity was grade 1 experienced by 6% of patients, and the maximum genitourinary acute toxicity was grade 2 in 63% of patients [16]. Our study aimed to add to the existing literature, and provide a more recent investigation on the acute toxicities associated with sHDR-BT.
Material and methods
Patient cohort
Patients consented to sHDR-BT as a standard of care offered at the study institution. A prospective database of all patients treated with sHDR-BT at a single large volume, tertiary cancer center was analyzed retrospectively. The database contained all relevant clinical and dosimetric information, including Common Terminology Criteria for Adverse Events (CTCAE) toxicity scoring for all patients. To be considered for sHDR-BT, patients were required to have experienced biochemical failure according to the Phoenix definition, after having received prior radical radiotherapy treatment with either EBRT or brachytherapy, or combination treatment [17, 18]. Standardized workup after biochemical failure included standard bloodwork and either of CT imaging of the chest, abdomen, and pelvis and a bone scan, or PSMA PET-CT in those with an access. Once localized disease was confirmed, further examination included a dedicated 3T MRI of the prostate, and trans-rectal ultrasound-guided systematic and targeted biopsies of the prostate. All pathologic specimens underwent centralized review prior to establishing the diagnosis of recurrent disease. One patient, with a prior diagnosis of castrate resistant but localized disease that was not responsive to darolutamide, was treated with sHDR-BT after tumor board review identified no other options for his management.
For all patients, follow-up at 1, 3, and 12 months post-treatment, and then yearly thereafter was performed, and included review of CTCAE toxicity, prostate specific antigen (PSA), testosterone levels, and current clinical status. The current study was approved by the Health Research Ethics Board of Alberta – Cancer Committee (approval number: HREBA.CC-23-0141_MOD1).
Treatment characteristic
Standard treatment recommendation included 2 years of ADT with three months of neoadjuvant treatment, followed by two, once weekly fractions of sHDR-BT and 21 months of adjuvant ADT. ADT agents used consisted of either leuprolide with 30 days of bicalutamide or degarelix (in patients with known coronary artery disease, peripheral vascular disease, or stroke) [19]. This treatment regimen was adapted during the COVID-19 pandemic due to limitations of operating room (OR) availability, and several patients received longer durations of neoadjuvant ADT.
Salvage HDR-BT was restricted to one of two approaches. First approach: 27 Gy in 2, once a week fractions localized to the biopsy proven prostatic regions of disease for patients having received prior brachytherapy treatment. Second approach: 10.5 Gy in 2, once a week fractions to the whole prostate with integrated boost(s) of 27 Gy in 2, once a week fractions to the biopsy proven prostatic regions of disease for patients having received only external beam radiotherapy treatment in the past. For either technique, transperineal needle implantation was performed under trans-rectal ultrasound guidance and spinal anesthetic. After needle implantation, continuous axial ultrasound image sets were used in Oncentra Prostate® to reconstruct the needle positions, and delineate all target contours and organs at risk, including the rectum and urethra. For MR-based nodules, which contained biopsy proven disease, cognitive fusion was applied to delineate boost volumes. No clinical target volume (CTV) margin was used on these nodules. For sites of biopsy proven disease, contours were at the discretion of treating physician, but often included the entire biopsy region (e.g., the right apex). All dominant intra-prostatic nodule contours were trimmed by 2 mm from the urethra (Figure 1). Plans were generated according to parameters established by Murgic et al., and with emphasis placed on meeting organ at risk dose volume constraints [20]. The constraints used are listed in Table 1. Treatment immediately followed planning using a technique described by Elangovan et al. [21].
Fig. 1
Example contours and isodose distributions for A) patient receiving sHDR-BT after external beam radiotherapy monotherapy, and B) patient receiving sHDR-BT after low-dose-rate prostate brachytherapy monotherapy. Contoured structures include the intra-prostatic nodule, prostate, urethra, and rectum. Isodoses are relative to 13.5 Gy
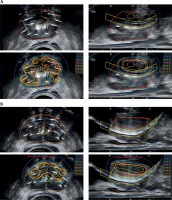
Table 1
Planning guidance constraints for salvage high-dose-rate brachytherapy (sHDR-BT) treatments for localized prostate cancer recurrence. Constraints are per fraction with an intent for 2 fractions to be delivered
Statistical analysis
Descriptive statistics were utilized to describe the cohort. For continuous variables, median and interquartile ranges were applied, whereas for discrete variables, descriptions included absolute count and proportions. Mann-Whitney-Wilcoxon test was used for comparisons between discrete variables. Comparisons between categorical variables were performed with Freeman-Halton expansion of Fisher’s exact test [22]. P-values of < 0.05 were considered statistically significant. All analyses were performed using R programming language version 4.0.0 (www.r-project.org).
Results
Seventeen patients were treated during the studied period. The median (interquartile range) age prior to sHDR-BT was 68 (66-74) years. At initial diagnosis, 13 (76%) patients had T1 or T2 disease (Table 2). Eleven (64%) patients had Gleason grade group (GG) 1 or 2 disease. The median PSA was 8 (6-12) ng/ml prior to initial therapy.
Table 2
Diagnostic and staging information at the time of initial diagnosis and prior to first radiotherapy treatment and at the time of salvage high-dose-rate brachytherapy (sHDR-BT) for 17 patients treated with sHDR-BT
The initial treatment for the entire prostate gland was as follows: 8 (47%) patients received EBRT monotherapy (74-78 Gy), 1 (6%) patient received EBRT (46 Gy) and low-dose-rate BT (LDR-BT) (110 Gy), and 8 (47%) patients received LDR-BT monotherapy (144 Gy). Elective nodal irradiation (46 Gy) with EBRT was applied in four (24%) patients. Nine (53%) patients received androgen deprivation therapy as part of their initial treatment. The postinitial therapy PSA nadir was 0.4 (0.2-1.2) ng/ml.
The median time from initial radiotherapy to biopsy confirmation of recurrent disease was 62 (52-106) months. At the time of relapse, the median PSA was 4.8 (2.8-8.3) ng/ml (Table 2). On dedicated 3 Tesla prostate MR after diagnosis of biochemical recurrence, 2 (12%) patients had no evidence of disease within the prostate on MRI, but had biopsy-positive disease. Of those patients with nodules identified within the prostate, the median size was 2 (1-2) cm. One (6%) patient had the bladder and one (6%) patient had both the bladder and levator ani muscle involvement at the time of relapse. On repeat biopsy, 12 (70%) patients had GG2-3 disease, and 5 (30%) had GG4-5 disease.
All the patients (100%) completed 2 of 2 fractions of sHDR-BT, and all the patients (100%) received neoadjuvant and/or adjuvant ADT with sHDR-BT. The dosimetry achieved at the time of sHDR-BT is presented in Table 3.
Table 3
Dosimetry achieved during first and second fraction of salvage high-dose-rate brachytherapy (sHDR-BT) for patients with intra-prostatic relapse of prostate cancer after initial radiotherapy treatment
At baseline prior to sHDR-BT, 8 (47%) patients reported that they were bothered by their lower urinary tract symptoms. The median American Urological Association (AUA) score prior to sHDR-BT was 7 (3-18) (Table 4). One patient (6%) reported hematuria, 2 (12%) experienced hematochezia, and 3 (18%) reported irregular bowel function at baseline prior to sHDR-BT.
Table 4
AUA symptom scores before and after salvage high-dose-rate brachytherapy (sHDR-BT) in cohort of 17 patients
The median post-sHDR-BT follow-up time was 20 (13-24) months. One (6%) patient had PSA recurrence post-sHDR-BT after testosterone recovery, and was re-started on systemic therapy. One (6%) patient had locally progressive disease outside of the treatment volume, with further erosion of the previously involved levator ani muscle.
The median AUA score at 4 weeks post-sHDR-BT was 13 (8-21), and was not significantly different from pre-sHDR-BT scores (p = 0.21). Table 4 shows a full comparison between pre- and post-sHDR-BT AUA symptom assessments. On genitourinary CTCAE toxicity scoring, there was no CTCAE grade 3 or higher toxicity, but 13 (77%) patients experienced at least one CTCAE grade 2 toxicity. The most common grade 2 genitourinary toxicity was bladder spasming, i.e., 10/17 (59%) and 10/17 (59%) at 1 and 3 months post-sHDR-BT, respectively (Table 5). On gastrointestinal CTCAE toxicity scoring, there was no CTCAE grade 3 or higher toxicity. The only grade 2 gastrointestinal toxicity encountered was anal pain, i.e., 1/17 (6%) at 3 months post-sHDR-BT (Table 6). In addition, there were no reported anal/rectal fissure, colitis, fistula, fecal incontinence, or bowel perforation toxicities at 1 or 3 months post-treatment.
Table 5
Common Terminology Criteria for Adverse Events (CTCAE) reporting genitourinary toxicity scores at time-points after salvage high-dose- rate brachytherapy (sHDR-BT)
Table 6
Common Terminology Criteria for Adverse Events (CTCAE) reporting gastrointestinal toxicity scores at time-points after salvage high- dose-rate brachytherapy (sHDR-BT)
Discussion
In this retrospective analysis of 17 patients, we identified minimal acute gastrointestinal (GI) toxicities, and 3 quarters of patients experienced acute genitourinary (GU) toxicities. The median follow-up was 20 months, and biochemical response was generally achieved, with one patient experiencing PSA recurrence and another patient with locally progressive disease. Overall, the study showed promising results of acute toxicity in sHDR-BT.
After primary radiotherapy treatment for prostate cancer, the salvage options available to patients are often limited by toxicity. However, in addition to sHDR-BT, physicians and patients often consider prostatectomy, cryotherapy, low-dose-rate brachytherapy, and high-intensity focused ultrasound (HIFU). Of note, life-long androgen deprivation therapy is often employed, but is a non-curative option [23]. Amongst these treatments, salvage prostatectomy has some of the longest reported data [24, 25]. With the advent of robotic-assisted salvage prostatectomies, the rates of rectal injury (2%) and frank incontinence (32%) have decreased [26]. In their recent review, Grubmuller et al. reported rates of erectile dysfunction between 87% and 100%, and rates of intermittent urinary incontinence were between 27% and 77% [25]. Considering the advanced expertise required to perform salvage prostatectomy and the associated risks of treatment, it is often not recommended to patients. Low-dose-rate brachytherapy has previously been studied by Kollmeier et al. and Crook et al. [27, 28]. Although this technique was well-tolerated overall, there were reported instances of CTCAE grade 3 urinary retention, uretero-rectal fistula, incontinence, and proctitis (all reported as 1-2%). However, obstructive urinary symptoms were common. With this in mind, low-dose-rate brachytherapy is still considered a viable salvage option in centers with expertise. HIFU and cryotherapy have also been studied as salvage treatments post-radiotherapy [29-33]. Despite comparable local control, patients should be counseled about HIFU’s overall investigative nature, with preliminary data suggesting high rates of urethro-rectal fistula (3-10%) in addition to the risks of stricture and urinary retention. Salvage cryotherapy has been associated with urinary incontinence (10-30%) and fistula (3-5%), in addition to a 90-100% rate of erectile dysfunction. Both of these options seem to provide reasonable local control, but perhaps more exciting is their potential in focal salvage treatments [31]. In this capacity, we would argue that sHDR-BT may also prove useful as a tool for future study.
This study adds to the existing literature on sHDR-BT, and demonstrates that it has limited acute morbidities, as seen from our cohort. In the present study, the toxicities encountered were managed with over-the-counter analgesics (pain), alpha antagonist (obstructive urinary symptoms), antimuscarinics, or β3 agonists (refractory obstructive urinary symptoms) as well as Kegel exercises, antimuscarinics/β3 agonist trials, and/or pads (incontinence). The readily available nature and reasonable side effect profile to these medication classes suggest that sHDR-BT acute complications may be easily managed. Our study also shows comparable acute GI results to studies in the literature, as seen in Table 7 [14-16, 34-46]. Ménard et al. [11] used MRI-only or MRI-TRUS guidance sHDR-BT, and reported similar toxicity outcomes. In their cohort of 88 patients, the total dose given ranged from 22-26 Gy, delivered in 2 fractions. They observed no grade 3 or higher GI and GU toxicities attributed to salvage brachytherapy. Three (3%) patients reported grade 2 GI toxicity, which is comparable with our findings of one (7%) patient that reported grade 2 GI toxicity. They also reported a higher number of patients with grade 2 GU toxicities compared with grade 2 GI toxicities, which is in line with our findings. Corkum et al. [35] investigated a cohort of 30 patients treated with a dose of 27 Gy, divided into 2 fractions. They reported that 23 (76.7%) patients experienced a maximum acute GU toxicity of grade 2, and 2 (6.7%) experienced a maximum acute GI toxicity of grade 2. This is comparable with our findings of one (7%) patient having a maximum acute GI toxicity of grade 2, and 13 (77%) patients having a maximum acute GU toxicity of grade 2. Table 7 provides more details on the current studies in the literature. Overall, in studies that reported acute GI and GU toxicities, the maximum acute GI toxicity experienced was grade 2, and the maximum acute GU toxicity was grade 3. Furthermore, the rates of acute GU toxicities were higher than the rates of acute GI toxicities, which is consistent with the present study.
Table 7
Literature review of salvage-high-dose-rate brachytherapy studies
| Study [Ref.] | Treatment (years) | No. of patients | Inclusion criteria for treatment | HDR-BT | Time | Outcome reported | Outcome | Toxicity measure | Max. acute GI | Max. acute GU | Max. late GI | Max. late GU |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chen et al. [34] | 1998-2009 | 52 | BF | 6 Gy × 6 fx. | 5 years | FSBF | 51% | CTCAE | Grade 1 (NR) | Grade 3 (2%) | Grade 2 (4%) | Grade 3 (2%) |
| Chitmanee et al. [14] | 2013-2018 | 50 | BF | 19 Gy × 1 fx. | 3 years | FSBF | 46% | CTCAE | Grade 2 (8%) | Grade 2 (54%) | Grade 2 (8%) | Grade 3 (10%) |
| Corkum et al. [35] | 2012-2019 | 30 | BP | 13.5 Gy × 2 fx. | 3 years | FSBF | 61.8% | CTCAE | Grade 2 (6.7%) | Grade 2 (76.7%) | Grade 2 (NR) | Grade 3 (3%) |
| Jiang et al. [36] | 2003-2011 | 22 | BF | 10 Gy × 3 fx. | 5 years | FSBF | 45% | CTCAE | Grade 2 (9%) | Grade 1 (NR) | Grade 2 (9%) | Grade 3 (9%) |
| Kissel et al. [37] | 2013-2020 | 64 | BF | 12 Gy × 2 fx. 13 Gy × 2 fx. | 2 years | DFS | 58% | CTCAE | Grade 1 (NR) | Grade 3 (1.5%) | Grade 3 (1.5%) | Grade 3 (1.5%) |
| Kollmeier et al. [38] | 2003-2015 | 61 (HDR) | BP | 8 Gy × 4 fx. 7 Gy × 4 fx. 11 Gy × 2 fx. | 3 years | FSBF | 60.1% | CTCAE | Grade 2 (NR) | Grade 2 (NR) | Grade 3 (NR) | Grade 3 (13%) |
| Lee et al. [39] | 1998-2005 | 21 | BP | 6 Gy × 6 fx. | 2 years | FSBF | 89% | CTCAE | Grade 2 (14.2%) | Grade 3 (NR) | NR | Grade 3 (14.2%) |
| Lyczek et al. [40] | 1999-2008 | 115 | BF | 10 Gy × 3 fx. | 5 years | FSBF | 46% PSA ≤ 6, 18% PSA > 6 | RTOG | NR | Grade 3 (2.6%) | NR | Grade 4 (3.5%) |
| Maenhout et al. [15] | 2013-2016 | 17 | BP | 19 Gy × 1 fx. | 1 year | FSBF | 92% | CTCAE | NR | Grade 2 (11.8%) | NR | Grade 3 (5.9%) |
| Murgic et al. [41] | 2012-2015 | 15 | BP | 13.5 Gy × 2 fx. | 3 years | FSBF | 61% | CTCAE | Grade 1 (20%) | Grade 2 (93.9%) | Grade 2 (13%) | Grade 3 (6.7%) |
| Slevin et al. [16] | 2015-2018 | 43 | BP | 19 Gy × 1 fx. | 3 years | FSBF | 41.8% | CTCAE | Grade 1 (14%) | Grade 2 (63%) | Grade 1 (14%) | Grade 3 (2%) |
| Tharp et al. [42] | 2001-2006 | 7 | BP | No consistent regimen | 58 months | DFS | 71% | CTCAE | NR | NR | NR | Grade 3 (29%) |
| Van Son et al. [43] | 2013-2017 | 50 | NR | 19 Gy × 1 fx. | 2.5 years | FSBF | 51% | CTCAE | Grade 2 (NR) | Grade 2 (52%) | Grade 2 (6%) | Grade 3 (2%) |
| Van Son et al. [44] | 2013-2019 | 150 | NR | 19 Gy × 1 fx. | 20 months | Toxicity | NA | CTCAE | Grade 2 (2.1%) | Grade 2 (20.8%) | Grade 2 (4.7%) | Grade 3 (3.9%) |
| Wojcieszek et al. [45] | 2008-2014 | 83 | BP | 10 Gy × 3 fx. | 5 years | FSBF | 67% | CTCAE | Grade 1 (6%) | Grade 3 (1%) | Grade 1 (6%) | Grade 3 (13%) |
| Yamada et al. [46] | 2007-2011 | 42 | BP | 8 Gy × 4 fx. | 5 years | FSBF | 68.5% | CTCAE | NR | Grade 2 (40%) | Grade 2 (14%) | Grade 3 (9.5%) |
| Present study | 17 | BF | 13.5 Gy × 2 fx. 5.25 Gy × 2 fx. | 3 years | FSBF | 88.2% | CTCAE | Grade 2 (6%) | Grade 2 (77%) | NR | NR |
This study did include the treatment of 2 patients with locally advanced recurrent prostate cancer. Because one case developed subsequent progressive disease outside of the brachytherapy field, other palliative options may be more appropriate than focal treatments in these scenarios.
In this study, the approach differed from existing literature by utilizing only cognitive fusion with an MRI acquired pre-brachytherapy. Notably, there was no image registration conducted in the unshielded operating room, and patients were transported to the treatment room for the delivery of radiation. The details regarding this method is described in Elangovan et al. [21]. It is important to acknowledge that this utilization of cognitive fusion requires significant expertise, and does carry a higher degree of inaccuracy than provided by an MR-based planning process. In order to compensate for this, the authors were more generous in their contouring of intra-prostatic nodules, which may have led to overtreatment within regions of the prostate. Notwithstanding, the toxicities were low, which suggest it may be a safe practice. Otherwise, when considering fractionation for use in sHDR-BT, the authors considered the primary prostate treatment data presented by Morton et al. who suggested that single fraction HDR-BT can be inferior, and a possible radiobiologic rationale for this may be re-oxygenation [47]. With this in mind however, a variety of fractionation schedules have been employed (Table 7). The authors chose to pursue a two-fraction regimen to alleviate pressures on their operating room resources; however, maybe in time, more extended fractionation schedules would prove superior.
Additionally, it is important to note that all patients at the study center received a standardized course of ADT of 2 years duration. The rationale for this practice was driven by the radiobiologic argument that inherent radioresistance should be present in prostate cancer cells surviving an initial radiotherapy treatment. The use of ADT in this circumstance may induce radiosensitivity in these cells or at least force cellular senescence, and improve curability of the disease. Notably, this reasoning is primarily informed by data showing improved biochemical and metastatic disease-free survival control rates in patients receiving external beam radiotherapy as an upfront treatment or salvage therapy after prostatectomy [48-50]. To date, although ADT is commonly considered in the setting of brachytherapy for prostate cancer, the exact benefit has not been clearly defined and is instead estimated using retrospective analyses [51]. The primary concerns with analyses such as this are the doses used in HDR-BT, which are far beyond the predicted required dose for a 99% probability of sterilizing an intra-prostatic tumor. One assumption to rectify the apparent contradiction is that ADT may be improving tumor control in the periprostatic fat tissue or single cells within the lymphatic drainage of the prostate. In the setting of sHDR-BT, there is a variety of practices around the duration of ADT used. Given that the overall rate of localized failure for prostate cancer post-radiotherapy is low, the use of ADT in salvage treatment for prostate cancer should be explored in future pooled analyses. Until such a time, when data would be available to analyze individual cases, brachytherapists should consider these arguments when engaging with patients in joint decision-making.
Our study is retrospective in nature, which inherently introduces bias in data analysis. Furthermore, the absence of randomization prevents from definitively establishing the treatment’s benefits. Another limitation stems from the small sample size, as the procedure is applicable to a limited subset of eligible patients. Given that the method used to perform brachytherapy was unique to the study center and possibly has a higher risk of uncertainty in needle position [21], our approach included obtaining additional ultrasound images in the treatment room prior to initiating treatment, to ensure that the catheters were in the same position as the planning ultrasound. However, despite this imaging protocol, the absence of a control group comparing our approach with the traditional method remains a limitation, impacting the study’s generalizability. A further limitation of this study is due to the inclusion of two distinct treatment groups in our study, thereby introducing inherent variability making it difficult to interpret the results and determine if the outcome is related to the procedure or other factors, such as treatment regimen.
A key strength of our study was the consistency of the data collection through the major time-points. The CTCAE and AUA scores were consistently collected in a highly regimented fashion and very well adhered to. Although the study had a short follow-up duration, our focus was primarily on acute toxicities, necessitating long-term data for comprehensive discussion of prolonged toxicity effects.
The present study adds to the existing literature and demonstrates that salvage HDR-BT may be a safe option for patients with recurrent prostate cancer. Relatively minor acute GI and GU toxicities were encountered, and no cases of CTCAE grade 3 or higher genitourinary toxicities were observed. As our study reported on the acute toxicities associated with sHDR-BT, it is important to recognize that the long-term outcomes are of equal importance, such as late toxicity outcomes and efficacy, therefore future studies in this area would be beneficial.


