The practice of administering intravenous (IV) fluids originated from the cholera pandemic in 1831, when doctors realized the impact of intravascular volume and electrolyte depletion in significantly dehydrated patients suffering from severe diarrhea [1].
Robert Lewis initiated the first IV infusion in a cholera patient whose condition improved as a result; however, it was not until the 19th century that IV saline management in cholera patients was widely accepted by the medical community. It was only during the 20th century, with the onset of the First World War, that its ability to save lives was tested [1].
Medicine has traditionally focused on therapies based on improving cardiac output. However, it has been shown in the last decade (Table 1) that this has had no impact on survival; the proposal to improve microcirculatory blood flow without unnecessary IV fluid therapy will ultimately avoid complications associated with medical malpractice (Table 2).
TABLE 1
Impact of fluid overload on the prognosis of critically ill patients
| Clinical trial | Year | Intervention | Methodology | Results |
|---|---|---|---|---|
| FEAST [66] | 2013 | Group 1: bolus 20 mL kg-1 saline 0.9% OR bolus 20 mL kg-1 albumin 5% OR maintenance fluids Group 2: bolus 40 mL kg-1 normal saline 0.9% OR bolus 40 mL kg-1 albumin 5% | Group 1: 3141 paediatric patients with no severe shock Group 2: 29 paediatric patients with severe shock | Mortality at 48 hours Increased mortality in the fluid bolus group RR = 1.45; CI 95%: 1.13–1.86; P = 0.003 |
| Positive fluid balance in sepsis [67] | 2015 | To study whether a positive fluid balance is an independent prognostic factor in patients with sepsis | n: 173 37 ICU’s | Positive fluid balance was an independent mortality predictor RR = 1.014 (1,007–1,022) per mL kg-1; P < 0.001 |
| Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome Meta-analysis [68] | 2016 | Compared conservative resuscitation to a liberal strategy in patient with sepsis and ARDS | 11 units with 2051 patients: adults and children | Neutral mortality Conservative strategy: increased days without MV and reduced length of stay in the ICU |
| DoReMi [69] | 2016 | Investigated the impact of daily fluid balance and fluid build-up on mortality in critically ill patients | 1734 patients from 21 ICUs from 9 countries | Mortality of 22.3% in patients with acute renal injury and 5.6% in those without acute renal injury (P < 0.0001) |
| CLASSIC [70] | 2016 | Restrictive fluid management vs. liberal | 152 adults with septic shock at ICU | Decreased mortality and decreased acute kidney injury |
| Fluid administration in severe sepsis and septic shock, patterns and outcomes: an analysis of a large national database [71] | 2017 | To identify the optimal fluid resuscitation strategy in the early hours of severe sepsis and septic shock, whether conservative or aggressive | 23,513 patients with severe sepsis and septic shock | In patients receiving volume resuscitation (5 to 9 L), mortality increased by 2.3% (95% CI: 2.0–2.5; P = 0.0003) for each additional litre above 5 L |
| ANDREWS [72] | 2017 | Early intravenous fluid therapy | 112 adults with septic shock in the ER | Increased hospital mortality was observed |
| Early resuscitation protocol on hospital mortality in adults with sepsis and hypotension: a randomized clinical trial [73] | 2017 | Early resuscitation for sepsis MAP and Hb goals during resuscitation: MAP > 65 mm Hg, Hb > 7 g dL-1 | Randomized clinical trial of 212 adults with sepsis and hypotension | Early resuscitation with intravenous fluids and vasopressors increased hospital mortality compared to regular care |
| FEDORA [74] | 2018 | Group 1: Guided via optimised stroke volume, mean blood pressure > 70 mm Hg, and cardiac index ≥ 2.5 L min-1 m-1 Group 2: Liberal therapy | 420 patients in total, 224 patients with guided therapy undergoing elective surgery | Neutral mortality Decreased complications in guided therapy (8.6% vs. 16.6%, P = 0.018) Decrease in hospital stay |
| SWIPE [75] | 2018 | Resuscitation fluid requirements and physiological responses with albumin 20% vs. albumin 4–5% | Controlled study in 321 adult patients requiring resuscitation with liquids in the first 48 hrs of ICU admission | Resuscitation with albumin 20% decreased resuscitation fluid requirements, decreased positive water balance, not associated with any evidence of damage compared to albumin 4–5% |
| RELIEF [76] | 2018 | Liberal vs. restrictive fluid management | 3000 adults post surgical abdominal major surgery; randomisation of 1490 patients to fluid restriction and 1493 patients to a liberal fluid strategy | A restrictive fluid regimen was not associated with a higher survival rate but was associated with a higher rate of acute kidney injury |
| Water overload index in children with sepsis and septic shock [77] | 2019 | Ratio of water overload and mortality in children with septic shock | Study in 263 children admitted with septic shock at pediatric ICU | Increased morbidity associated with water overload index > 10% (respiratory dysfunction, vasopressor requirement, and renal replacement therapy, as well as higher mortality) |
| FRESH [78] | 2020 | Evaluated the responsiveness to liquids as a result of passive leg lift | 13 hospitals included 124 patients with sepsis and septic shock Group 1: 83 patients systolic-guided resuscitation Group 2: 41 patients conventionally reanimated | Decreased need for kidney replacement therapy (5.1% vs. 17.5%, P = 0.04) Decreased days of mechanical ventilation (17.7% vs. 34.1%, P = 0.04) in group 1 compared to the usual attention |
TABLE 2
Complications of fluid overload
[i] CARS – cardio-abdominal renal syndrome, CIRCI – critical illness-related corticosteroid insufficiency, CPP – cerebral perfusion pressure, CVP – central venous pressure, EVLWI – extravascular lung water index, GEDVI – global end diastolic volume index, GFR – glomerular filtration rate, IAP – intra-abdominal pressure, ICG-PDR – indocyanine green plasma disappearance rate, ICP – intracranial pressure, IOP – intra-ocular pressure, MAP – mean arterial pressure, PaCO2 – partial pressure of carbon dioxide, PaO2 – partial pressure of oxygen, PaO2/FiO2 – oxygen arterial pressure/inspired fraction of oxygen, PAOP – pulmonary artery occlusion pressure, pHi – power of hydrogen, RVEDVI – right ventricular end diastolic volume.
IV fluids are usually an essential component in the management of critically ill hospitalized patients; however, excess fluid administration can cause harm, with an association between fluid accumulation, fluid overload (10% increase), and mortality [2–4].
As Paracelsus stated, “Nothing is without poison; it is the dose that makes the poison.” Starting in 2001, Emmanuel Rivers proposed the early application of IV fluids in patients with sepsis and septic shock, setting resuscitation targets with goals to be reached within the first 6 hours. The idea was to achieve adequate oxygen delivery (DO2) by modifying the determinants of cardiac output and haemoglobin saturation covering the patient’s demand, in order to improve microvascular perfusion. At that time, the potential damage caused by excessive fluid administration was yet to be examined [5].
In 2006, the SOAP study showed that fluid over-resuscitation is associated with increased mortality in sepsis patients [2]. Subsequently, the VASST study concluded with similar results, reporting that a positive fluid balance is an independent predictor for mortality [6]. Retrospective analyses of Micek and Sedaka reinforced the potentially harmful effects of over-resuscitation [4].
Despite these findings, it sometimes feels counter-intuitive to manage a hospitalized patient without a baseline IV solution running. While excessive fluid administration is now recognized to have harmful consequences, the administration of IV fluids even within an apparently safe therapeutic range has also been found to have “a dark side” [7]. Current evidence suggests that the risks of overzealous administration of resuscitation or maintenance fluids without a clear indication are outweighed by the benefits. Fluid toxi-city depends on the administered dose and composition of the fluid, the natural history of the disease, as well as the patient’s susceptibility [2].
The ADQI XII (acute dialysis quality initiating XII) research group proposed a conceptual framework for managing intravenous fluids based on risks related to any drug in order to raise awareness of the potential complications and recognizing the different phases of fluid therapy [8, 9]. Malbrain et al. [3] showed in a systematic review that restrictive fluid therapy decreases mortality and the time spent in the intensive care unit (ICU), regardless of the type of solution [2]. They suggested a similar framework illustrating the 4 dynamic phases of fluid therapy and the ROSE acronym (Resuscitation; Optimization; Stabilization; Evacuation) [3].
Analogously to antibiotic therapy, it is time for enhanced fluid stewardship [8, 10].
THE RATIONALE FOR INTRAVENOUS FLUID THERAPY
The NICE (National Institute for Health and Care Excellence) guidelines state that fluid therapy should be administered to patients whose daily fluid needs cannot be reached orally or enterally, and it should be discontinued immediately once this becomes possible [8]. IV fluid administration requires constant vigilance for complications associated with fluid overload. Clinical, radiological, and biochemical markers are currently available to assess fluid status and guide IV fluid administration [2, 11, 12]..
Before starting IV fluids, the 4 Ds proposed by Malbrain et al. should be considered (Table 3) [3, 8]. It is also important to recognize that the ideal fluid does not exist [13, 14].
TABLE 3
Intravenous fluid therapy considerations
INDICATIONS FOR INTRAVENOUS FLUID THERAPY
There are only 6 indications for IV fluids:
to replace fluids lost via enteral route or insensible losses (replacement solutions),
in patients unable to orally meet the daily needs for water, glucose, and electrolytes, maintenance solutions can be administered,
hypovolaemic shock (e.g. blood transfusion in the case of bleeding in trauma) [15],
to address daily caloric requirements (enteral or parenteral nutrition),
noticeable loss of intravascular volume or when there is a high suspicion thereof, e.g. in severe burns injury or gastrointestinal losses (resuscitation solutions),
for the administration of drugs (painkillers, antibiotics, etc.), also known as fluid creep (Figure 1).
Correction of dehydration: replacement fluids
Traditionally, IV fluids have been used to treat decreased intravascular volume in patients in whom the oral or enteral route cannot be used. These include gastrointestinal losses such as vomiting and diarrhoea, fever or hyperthermia, polyuria, lack of access to fluids or alterations in the thirst mechanism (e.g. in older adults), and second and third space losses. In these situations, replacement fluids can help to maintain acceptable blood flow, although the cause of hypovolaemia should be treated as a priority.
Clinical indications triggering the use of IV fluids are as follows: signs of dehydration (dry skin, sunken eyes, dry mucous membranes, loss of skin elasticity), hypotension with systolic pressure < 90 to 100 mm Hg, tachycardia with heart rate > 90 to 100 beats per minute, cognitive dysfunction, encephalopathy, mottled skin, delayed capillary filling > 2 s, cold extremities, and tachypnoea with breath rate > 20 breaths per minute [11, 16, 17]. Standard daily fluid needs are 1 mL kg-1 hr-1.
Electrolyte replacement is an important consideration when prescribing maintenance solutions. Electrolyte disturbances are a common cause for hospital admission and also a common occurrence during hospitalization. Any electrolyte disturbance with clinically significant implications is an indication for IV correction/replacement [18, 19]. The most common electrolyte disturbances (and their respective prevalence) are as follows: hyponatraemia (2.3–44%), hypernatraemia (1.1–4.4%), hypokalaemia (10.2–39%), hyperkalaemia (0.8–13%), hypercalcaemia (0.7–7.5%), hypophosphataemia (0.5–6.5%), hyperphosphataemia (1–17%), and hypomagnesaemia (1.7–8%) [20–24]. The association between hypernatremia levels and mortality is as high as 61% or up to 50% after correction. Another electrolyte disorder associated with poor prognosis is hyperkalaemia, often causes fatal arrhythmias, especially in patients with kidney or cardiovascular disease and diabetes mellitus [25].
Once the diagnosis has been established, the rate of correction should be considered: an infusion at an inappropriate rate may cause complications ranging from local (e.g. potassium IV phlebitis), through chronic systemic (e.g. osmotic sodium demyelination syndrome), to potentially fatal acute systemic complications (cardiac arrhythmias) [19]. The type and volume of fluid in which the electrolyte is to be diluted should also be taken into account, with care to avoid incompatible combinations and excessive volume [19, 25]. Standard daily needs for Na and K are 1.5 and 1 mmol kg-1 per day, respectively.
Covering daily fluid requirement: maintenance fluids
During hospitalization one of the most common healthcare activities regarding hydration revolves around measuring fluid balance; the accumulated fluid balance continues to be one of the eponymous numbers on nursing sheets. Simplistically, it equates fluid status with the input and output of fluids in patients. Insensible water loss is challenging to measure, i.e. the amount lost through respiration and skin.
It is acknowledged that accumulated fluid balance figures reported on nursing sheets may not accurately reflect the actual volume state of the patient [26, 27]. A positive or negative balance frequently leads to a presumption that the patient is overhydrated or dehydrated; this paradigm in clinical care will result in misconceptions regarding correct fluid prescription and administration.
The practice of using hypotonic maintenance fluids is based on the Holliday and Segar proposal from 1957 [20, 28] and was recently confirmed in healthy volunteers and critically ill patients [28]. The NICE guidelines recommend an initial prescription of maintenance fluid of 25–30 mL kg-1 per day of water [16].
Transfusion of blood products
In haemorrhagic shock, heart rate and arterial vascular tone are increased by compensatory neurohumoral responses in an attempt to maintain sufficient blood flow. However, in the event of severe bleeding, IV fluids maintain sufficient blood flow to the vital organs. Meanwhile, surgical or radiological interventions should be undertaken to stop the bleeding. Prompt recognition of the bleeding will allow initiation of adequate therapy as soon as possible, which can reduce the risk of potentially serious complications (e.g. consumption or dilutional coagulopathy, severe anaemia, cardiac ischaemia, bowel ischaemia, etc.) [29, 30]. The goal of resuscitation is to achieve adequate tissue perfusion and oxygenation while correcting coagulopathy [29]. IV fluids (other than blood) dilute clotting factors, decrease patient temperature, and potentially contribute to acidosis when only chloride-containing solutions (0.9% saline) are used; this will trigger a vicious cycle leading to tissue oedema and organ dysfunction. Eventual alteration of cellular mechanisms causing inflammation result in further complications including cardiac, respiratory, gastrointestinal, and immune dysfunction, hyperfibrinolysis, and increased mortality [15, 17].
Nutrition fluids
Nutrition plays a fundamental role in the management of critically ill patients; recent recommendations support the early introduction of oral, enteral, or intravenous nutrition [31]. Late initiation of nutrition is associated with increased morbidity, gastrointestinal dysfunction, malnutrition, and multiorgan failure. The amount of fluid administered to meet the daily nutritional requirements of a patient ranges between 250 and 500 mL on the first day, to nearly 1.5 L per day in adults, to achieve 25 to 30 kcal kg-1 per day. The daily glucose requirements are around 1–1.5 γ kg-1 per day. Malbrain et al. suggest that the fluids administered through nutritional supplementation should be taken into account within the patient’s total fluid balance [8, 32].
Resuscitation fluids
In an unprecedented manner in the history of medicine [33], the initial approach to fluid resuscitation in patients with sepsis had been arbitrarily mandated to consists of “at least 30 mL kg-1 of IV crystalloid fluid given within the first 1–3 h” despite a total lack of evidence to support this [34]. This “one size fits all” approach ignores the established literature on the deleterious effects of fluid resuscitation and basic physiology of distributive shock [35]. Many of these recommendations can be traced back to the 2001 Rivers study, which showed that the institution of an Early Goal-Directed Therapy led to decreased mortality amongst septic patients [5].
However, results from previous studies have failed to replicate this benefit [36], with 3 randomized controlled trials demonstrating worse outcomes in patients who received resuscitation with fluid bolus [37–39]. Furthermore, static haemodynamic measurements have been shown to be useless in predicting response from fluid administration, and they have been largely replaced by dynamic indicators of pre-load responsiveness [40]. Using these tools, fluid therapy should be tailored to the patient’s physiology rather than indiscriminate infusion of a predefined amount [41].
To avoid fluid overload, 2 complementary approaches may be used: restrictive fluid administration and the active removal of accumulated fluid. The concept of restrictive fluid administration relies on identifying and monitoring signs of fluid responsiveness during ongoing fluid administration, without signs of fluid intolerance. However, it should be emphasized that “fluid responsiveness” in a patient does not always mean that he/she is in need of fluids; giving fluids to a patient until he/she is no longer fluid responsive has not been shown to improve outcomes [40]. The ongoing CLASSIC trial will compare the differences between a liberal vs. restrictive fluid strategy in patients with sepsis. In its pilot feasibility trial, the restrictive fluid strategy led to reduced incidence of acute kidney injury [42].
Active removal of accumulated fluid should be considered simultaneously, given that fluid overload is unlikely to be avoided by conservative fluid strategy alone [8]. After the resuscitation, optimization, and stabilization phases of fluid resuscitation, aggressive fluid removal to achieve a negative fluid balance should be pursued by forced diuresis or ultrafiltration. This strategy has been called Late Goal-Directed Fluid Removal (LGFR) and should complement a Late Conservative Fluid Management (LCFM) in order to assure a return to euvolaemia [8, 42–46].
Fluid creep
Intravenous delivery of drugs requires fluid to be administered either intermittently or as continuous infusions (e.g. vasopressors, sedatives, etc.). Infused drugs (and the volume in which they are diluted) should be considered as part of the patient’s fluid balance.
Some drugs need a large amount of dilutional fluids (e.g. fluconazole, immunoglobulins, etc.). To avoid unnecessary fluid accumulation, drugs administered via continuous infusion should be diluted in the lowest volume possible [20]. A recent study showed that maintenance and replacement fluids accounted for 24.7% of the mean daily total fluid volume, far exceeding resuscitation fluids (6.5%), and were the most important sources of sodium and chloride. Fluid creep represented a striking 32.6% of the mean daily total fluid volume (median 645 mL [IQR 308–1039 mL]) [47].
COMPLICATIONS OF INTRAVENOUS FLUID THERAPY
Overview of secondary impact on end-organ function
“Fluids are not always life-saving”
Liberal IV fluid administration is associated with multi-organ complications secondary to water overload (Table 2) [3]. This is illustrated in Figure 2.
Pulmonary oedema
Normal lung water is about 500 mL in volume (< 7 mL per kg predicted body weight). The lungs need to be dry for normal gas exchange and surfactant function. Pulmonary oedema (increased extravascular lung water) can result from over-resuscitation and is associated with increased morbidity and mortality. Even small increases of approximately 300 mL of excess lung water has a dramatical impact on outcome [43, 48, 49]. In the presence of pulmonary oedema and P/F ratio < 100, fluid therapy needs to be modified to LCFM or LGFR [3]. A common difficulty encountered at the bedside is the early identification of pulmonary oedema [9].
Transpulmonary thermodilution is the current reference standard; a value of extravascular lung water index greater than 10 mL kg-1 PBW suggests pulmonary oedema [44]. Pulmonary ultrasound is a simple, non-invasive, and less expensive method. The identification of B-lines correlates with pulmonary oedema when compared to the reference standard [9, 45, 50].
Acute respiratory distress syndrome
Positive fluid balance is associated with deterioration of ventilatory mechanics and worse outcomes in patients with acute respiratory distress syndrome (ARDS) [46]. A meta-analysis published in 2017 assessed the effectiveness of conservative fluid resuscitation strategies compared to a liberal fluid strategy in adults and children with ARDS and sepsis; the conservative treatment group was associated with fewer days on a ventilator and shorter stay in the ICU [51]. Martin et al. found that negative fluid balances are associated with improvement in the PaO2/FiO2 relationship and haemodynamic parameters [51, 52].
Recently published guidelines recommend a conservative fluid resuscitation approach in ARDS patients, after demonstrating no benefit with liberal fluid management strategies [34, 53].
Interstitial oedema
The main mechanism of oedema formation is the degradation of the endothelial glycocalyx, which is responsible for regulating the permeability and displacement of fluids within the interstitial space. In fluid overload, the lymphatic system loses its ability to drain fluids and promote exchange, so tissue oedema occurs [54, 55]. During critical illness, physical and functional alterations of the glycocalyx lead to a pathological displacement of protein-rich plasma to the interstitium, which can occur even before the water overload affects the haemodynamics [56, 57]. This is referred to as global increased permeability syndrome or GIPS [8, 54] (Figure 2).
Coagulopathy and dilutional anaemia
Excessive administration of IV fluid results in dilution of plasma coagulation factors, alteration of fibrinogen levels [58], and a reduction in the haemoglobin concentration. The loss of capillaries full of erythrocytes, with a reduction in oxygen transport capacity and an ineffective supply of oxygen for the microcirculation, can cause organ dysfunction [59]. The consequent alteration in the haemodynamic state starts a vicious cycle, often resulting in the administration of even more unnecessary fluids [60, 61].
Electrolyte imbalances
Many of the IV solutions in use contain non-physiological concentrations of electrolytes. Unrestricted fluid therapy may lead to an unnecessary disturbance in electrolytes, such as hypo/hypernatraemia, hypo/hyperkalaemia, and hyperchloraemic metabolic acidosis, which, if not identified and treated, can result in organ damage (e.g. kidney injury) [62–64].
Sodium imbalance
A paediatric case-report study by Hoorn reported an association between fluid administration and hyponatraemia, although this was mainly attributed to the amount of fluid administered (causing a dilutional hyponatraemia) rather than the fluid composition [65]. In situations where renal dilution function is limited (e.g. elevated ADH levels), the infusion of isotonic fluids is associated with hyponatraemia by the desalinization phenomenon; this occurs due to renal excretion of the solutes infused with the rest of the water infused remaining in the intravascular space, thus worsening the hyponatraemia [66].
Potassium imbalance
Studies have demonstrated an increased risk of hyperkalaemia following administration of isotonic fluids compared with balanced solutions (even though balanced solutions contain potassium) – the serum potassium changes may occur via several renal and extrarenal mechanisms related to acidosis; however, no difference in clinical outcomes has been reported [67–69].
Hyperchloraemia
Chloride plays a predominant role in acid-base alteration. Sodium, potassium, chloride, magnesium, and calcium are strong ions that contribute to maintaining a pH of 7.35 to 7.45 under normal conditions [63]. Chloride undergoes free glomerular filtration with 99% reabsorption and excretion of approximately 180 mmol day-1. It is involved in the regulation of the Na–K ATPase pump, inducing the release of renin, vasoconstriction of the renal afferent artery, and reduction of glomerular filtration [70].
A study of healthy volunteers showed that hyperchloraemia is associated with a decreased mean rate of renal artery flow and infusion of renal cortical tissue with consequently decreased urine production [68, 71, 72]. Hyperchloraemic metabolic acidosis can induce vasodilation, decreased cardiac reactivity, decreased release of endogenous catecholamines, increased inflammatory response, and decreased splanchnic perfusion [68, 71, 73].
Infusion of saline solution at 0.9% can induce hyperchloraemia, which is related to metabolic acidosis, and is an independent mortality factor [48, 74].
Abdominal hypertension
Abdominal hypertension (IAH) is defined as a sustained increase in intra-abdominal pressure (IAP) equal to or above 12 mm Hg. A sustained IAP above 20 mm Hg with new-onset organ failure defines abdominal compartment syndrome (ACS) [75]. The major cause of secondary IAH and ACS is fluid overload in the setting of sepsis and capillary leak among other risk factors, e.g. increased intra-abdominal or intra-luminal contents and decreased abdominal wall compliance [76–78]. Fluid overload will lead to abdominal wall oedema (with diminished abdominal wall compliance), bowel oedema (leading to ileus), and venous congestion, hence increasing intra-abdominal volume causing a further increase in IAP. Eventually this may lead to increased pressures in other compartments, resulting in cardio-abdominal renal syndrome (CARS) [79, 80] and the polycompartment syndrome [81].
Subgroups with high risk of overhydration
Particular attention should be paid to patients at high risk of overhydration, e.g. those with cardiac, renal, or hepatic failure and nutritional disorders.
Patients with hepatic fibrosis have an increased portal circulation pressure, which can cause plasma leakage at the peritoneal level (ascites). This enhances hypoproteinaemia and in turn aggravates ascites and capillary leakage in a vicious cycle [82].
In patients with advanced chronic kidney disease, decreased filtration leads to fluid accumulation in the second and third space, which can account for up to a 4.5 kg increase in body weight. Correction of fluid accumulation can be achieved with diuretics; therefore, accurate assessment of volaemic status must be performed. A study comparing the effects of normal fluid balance vs. fluid overload in patients on renal replacement therapy for chronic kidney disease demonstrated higher mortality in overhydrated patients [83].
TRIGGERS TO STOP INTRAVENOUS FLUID THERAPY
The traditional approach has been to administer fluids until the patient is no longer fluid responsive. Fluid responsiveness is defined as a 15% increase in cardiac output after fluid resuscitation. However, this strategy may lead to fluid overload [8]. Although fluid overload is associated with increased morbidity and mortality, there are no clear parameters guiding the physician on when to stop fluid administration. Clinical and imaging variables suggesting the presence of interstitial oedema occur late.
Clinical assessment of fluid overload
Clinical parameters of fluid overload are non-specific and thus not useful to trigger deresuscitation. These include the following: altered mental status, increased hepatojugular reflux, orthopnoea, second and third space fluid accumulation, pitting oedema, altered capillary refill, increased jugular venous pressure, increased body weight, and a positive daily and cumulative fluid balance [11].
Biochemical parameters
Biochemical parameters of fluid overload (haemo-dilution) are again non-specific. These include the following: increased BNP and pro-NT-BNP, decreased colloid oncotic pressure, signs of infection and inflammation, increased CRP, decreased albumin and total protein levels, increased serum capillary leakage index (CRP divided by albumin), increased urine albumin over creatinine ratio, presence of AKI (urinalysis), and dilutional anaemia.
Central venous pressure and pulmonary artery occlusion pressure
In 1984 Shippy [84] conducted an analysis of fluid therapy and its relationship to variables such as central venous pressure (CVP), concluding that they do not adequately reflect the volume status of critically ill patients. Therefore, they are not currently recommended for guiding fluid removal [85, 86].
In patients without structural pathology of the right cardiac cavities (e.g. tricuspid disease), CVP reflects right ventricular pressure. This association was initially taken as a strategy to select patients responding to fluid administration based on baseline CVP values and the dynamics of CVP changes after fluid bolus [8]. However, it has been shown that the isolated use of an absolute CVP value does not predict whether a patient will be a fluid responder [35]. At best, CVP can only be considered as a guide to stop IV fluids if it is above normal values (6–8 mm Hg) or if it rises by > 5 mmHg after a fluid bolus (4 mL kg-1 15 min) [62]. The VASST study showed increased mortality associated with fluid overload and high CVP (> 12 mm Hg) [6, 85–87]. A high CVP is also an independent predictor for worsening renal function, not only in patients with decompensated heart failure [88] but also in sepsis [89].
Bioelectrical impedance analysis
Bioelectrical impedance analysis (BIA) is a non-invasive technique used to estimate body composition. It is an inexpensive test [93], with studies demonstrating good correlation with values obtained through the gold standard deuterium dilution method (r = 0.996) [94].
The technique has been validated in different patient populations and clinical scenarios for fluid status monitoring [95–97]. Kammar-Garcia et al. [98] showed in a prospective observational study of patients admitted to the emergency department that fluid overload as evaluated by bio-electrical impedance vector analysis (BIVA) was significantly related to mortality, and that failure to clinically determine fluid status at time of admission can lead to a mishandling of fluid management in critically ill patients. Fluid overload may already be present at a subclinical level, even before starting IV fluids; BIA evaluation of fluid status at (and during) admission can help guide fluid management [99]. Body weight is often used as a crude measure of fluid balance; however, this does not take into account the skeletal muscle wasting associated with critical illness [100, 101], and therefore cannot provide an accurate reflection. The mortality risk associated with fluid overload (as determined by BIVA) has been documented in hospitalized patients [102] at hospital discharge and at readmission of patients with heart failure, critical illness and those on total renal replacement [103]. Therefore, BIVA, as a non-invasive, low-cost, rapid, and easy technique, could replace accumulated fluid balance as a more accurate and objective parameter of fluid and muscle shift balance (Figure 3).
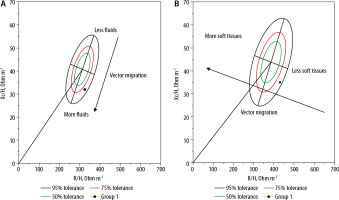
FIGURE 3
RXc graph for male (A) and female (B), with bioelectrical impedance vector analysis (BIVA). R – resistance (ohms) measured at 50 kHz; Xc – reactance (ohms) measured at 50 kHz; H – height expressed in metres, 50, 75, and 95% tolerance ellipse are shown (green, red, and black ellipse, respectively). Vector migration from less to more body fluids in the male graph and less to more soft tissues in the female graph are shown for schematization Source: Image courtesy of Eduardo Argaiz performed at National Institute of Medical Sciences and Nutrition Salvador Zubiran
Imaging techniques
Traditionally, plain chest radiographs were used to assess for signs of fluid overload, including the presence hilar congestion, pleural effusion, Kerley-B lines, etc. However, the subjectiveness of interpretation and static nature of this modality limit its usefulness as a monitoring tool. Critical care ultrasound has superseded plain radiographs as the imaging tool of choice for identification of fluid status. The ease of use, sensitivity for pleural and peritoneal fluid, as well as accessibility for repeated imaging make it ideal for monitoring the dynamic process of fluid resuscitation.
The diameter and variability of the inferior vena cava and internal jugular vein
Measuring the diameter and variability of the inferior vena cava (IVC) and the internal jugular vein (IJV) is another proposed tool for assessing fluid status. A significant change in the diameter of these large vessels during inspiration may be associated with an adequate response to volume; conversely, a variation in the diameter of ICV or IJV < 12% in mechanically ventilated patients or between 36 and 50% in spontaneously breathing patients suggests that no benefit will be gained from further intravenous fluid administration [90, 91].
It has been observed that the normal maximum diameter of the IVC ranges from 1.9 to 2.1 cm; patients presenting with an IVC diameter close to this, with minimal or no variation during the respiratory cycle, do not benefit from IV fluids [44]. The use of the IVC collapsibility index does have some limitations, including inter-observer differences, high rates of false positives, and mild-to-moderate positive predictive value, as discussed in the review paper by Via et al. [92]. These include the use of high external PEEP levels, use of non-invasive ventilation, assisted spontaneous breathing (ASB) with low tidal volume, the presence of auto-PEEP, right ventricular dysfunction, tamponade, abdominal hypertension, mechanical obstruction, respiratory variations, or right ventricular myocardial infarction.
Ultrasonographic evaluation of systemic venous congestion
Pathological elevation of CVP is an important factor for the development of congestive organ damage [88]. In patients with congestive heart failure, the main haemodynamic parameter associated with the development of acute renal injury is the increase in CVP and not the cardiac index (CI) [89]; this is also true in patients with sepsis [104]. Similarly, the severity of congestive liver disease correlates with elevation of right atrial pressure (and hence CVP), not with CI [105].
Organ damage associated with congestion occurs secondarily to the retrograde transmission of CVP to the parenchymatous veins, which alters the venous flow pattern [106]. For example, the transmission of CVP into intra-renal veins generates renosarca and a decrease in renal perfusion pressure (local renal compartment syndrome) [107]. Ultrasound allows direct assessment of blood flow at the organ level using Doppler techniques [108]. Several groups of researchers have found strong associations between organic venous flow disturbances and important outcomes such as acute kidney injury [109], congestive encephalopathy [110], and mortality [111].
The assessment of organ venous congestion should begin by evaluating congestion at the systemic level, i.e. the volume and collapse of the IVC, as previously described. This assessment, performed in the short axis with cephalo-caudal views, provides an impression of the volume of a 3-dimensional structure [112]. An IVC diameter greater than 2 cm with less than 20% collapse on inspiration is considered the first sign of venous congestion [108].
Next, the flow pattern in the portal vein is assessed. Generally, the portal vein is protected from CVP by the resistance generated by the hepatic sinusoids; however, when CVP is pathologically raised, the retrograde pressure can reach the splanchnic pool and affect the venous flow pattern. Therefore, normal portal flow is continuous, but this becomes pulsatile in patients with severe venous congestion. A pulsatile rate greater than 30% is considered moderate congestion, and over 50% is considered severe. Similarly, intra-renal venous flow assessment distinguishes flow patterns associated with congestion. Continuous renal flow is considered normal; this becomes pulsatile, biphasic, and single-phase in order of severity of venous congestion (Table 4) [111].
TABLE 4
Grading table for assessment of Venous congestion with point-of)-care ultrasound VEXUS = venous congestion assessment with ultrasound (adapted with permission from Rola P. et al book “Bedside Ultrasound: a primer for clinical integration” [129])
The combination of these alterations provides not only an estimation of CVP but also an idea of its impact on end organs [108]. In our view, the presence of venous congestion is a powerful argument against the administration of IV fluids, which may exacerbate congestive organ damage regardless of the presence of dynamic volume response predictors. However, there are exceptions (cardiac tamponade, tension pneumothorax, severe chronic pulmonary hypertension), and therefore no decision should be made based on an individual parameter. Of particular importance, in patients with severe venous congestion (portal pulsatility > 50%), the use of diuretics may improve organ function. The diagnosis of venous congestion should not be limited to assessment for its presence and severity; identification and treatment of the underlying cause (e.g. volume overload, congestive heart failure, cardiac tamponade, etc.) are crucial [113].
Figure 4 shows an example of a non-congestive patient and one with severe venous congestion.
FIGURE 4
A) Patient not congestive. Left: Short-axis display of the lower vena cava at the level of the origin of the hepatic veins. IVC diameter: 9 mm. Right: Pulsed Doppler of the portal vein showing minimal pulsatility (continuous flow). B) Patient with severe congestion. Left: Short-axis display of the lower vena cava at the level of the origin of the hepatic veins. IVC diameter: 34 mm. Note also the dilation of the supra-hepatic veins. Right: Pulsed Doppler of the portal vein showing 100% pulsatility ([Vmax – Vmin/Vmax] × 100)
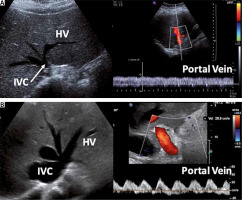
The role of venous congestion in the development of worsening organ function in patients with fluid overload may explain the improvement in renal function following deresuscitation (either via diuretics or ultrafiltration), as characterized by echocardiographic signs of fluid overload on IVC, portal, hepatic, and renal veins (i.e. sustained distention) (Table 4) [114].
Focused echocardiography
Echocardiography can provide objective data on the patient’s volume status and the cardiac response to fluids [61]. A velocity time integral (VTI) > 17 cm infers a normal systolic volume; a change < 12% with fluid administration or passive leg elevation is associated with lack of response to IV fluid administration.
There exist different ultrasound data indicators of right ventricular (RV) failure; these are important because a dysfunctional RV will poorly tolerate preload increases and may paradoxically decrease cardiac output due to ventricular septal interdependence. RV dysfunction is suspected when the RV: LV area increases (RV/LV) > 0.7 to 1 or the tricuspid annular systolic displacement (TAPSE) value is < 8 mm. Caution is needed when interpreting TAPSE in the presence of associated RV failure, chronic pulmonary hypertension, and invasive mechanical ventilation. Left ventricle (LV) function can be evaluated via the ejection fraction (EF), which is the percentage of end-diastolic ejected volume during each heartbeat; an LVEF < 55% suggests inadequate mobilization of blood volume and a tendency for pulmonary and systemic congestion. Different parameters that can help guiding de-escalation of intravenous fluid therapy are listed in Table 5.
TABLE 5
Variables that suggest stopping intravenous fluid therapy
* A small amplitude of the systolic waveform is associated with a decreased systolic volume; conversely, a large amplitude correlates with vasodilation and obviates the need of fluid resuscitation. HR – heart rate, NT-proBNP – N-terminal pro–B-type natriuretic peptide, ScvO2 – central venous of carbon dioxide saturation, SvO2 – mixed venous oxygen saturation, VTI – velocity time integral, ∆VTI – delta velocity time integral, RV/LF – right ventricular/left ventricular, TAPSE – tricuspid annular plane systolic excursion, EF – ejection fraction, PPV – pulse pressure variation, SVV – stroke volume variation, PVI – Pleth variability index, CVP – central venous pressure, ∆CVP – delta central venous pressure.
FLUID REMOVAL
When a patient does not show fluid-responsiveness on assessment using clinical or dynamic parameters, interventions should be initiated to actively avoid fluid overload, given its possible consequences. Alternative methods are needed to maintain adequate organ perfusion, e.g. early use of vasopressors [113, 115].
As stated above, there are 2 strategies to avoid fluid overload: restriction of IV fluids (prevention) and removal of excess fluid using diuretics or renal replacement therapy with ultrafiltration (intervention) in haemodynamically stable patients [113]. These strategies can be used concurrently.
Achievement of negative fluid balance using deresuscitation strategies within the first 3 days of admission has been associated with decreased mortality compared to that seen in patients who remained in positive fluid balance. Restrictive fluid therapy also resulted in fewer days on mechanical ventilation [43].
In a meta-analysis, Chen et al. demonstrated the association of an early furosemide stress test with a loop-diuretic (furosemide)-identified tubular reserve. A positive response in an AKI II subgroup was associated with decreased requirement for renal support and overall mortality rate [116].
Current evidence shows that the greatest sources of fluid accumulation are maintenance solutions (to cover basic daily needs) and fluid creep. This suggests that positive fluid balance is a variable driven by practice, and that it is therefore modifiable [51].
This is especially the case in patients with sepsis (and capillary leak) where higher extravascular lung water values have been reported even without the presence of overt ARDS, suggesting subclinical acute lung injury; and with beneficial effects after deresuscitation strategies [117]. Fluid accumulation in the early course should be avoided in patients with sepsis and ARDS. A multivariate model showed that a more positive fluid balance on the third day was associated with longer durations of ICU admission and mechanical ventilation in survivors, while early fluid removal at this point was associated with better outcomes [40]. Furthermore, in a retrospective matched case-control study of 114 patients on mechanical ventilation with acute pulmonary injury, Cordemans et al. found that the application of the multimodal fluid restriction strategy had beneficial effects. The so-called PAL-treatment is an approach that combines high levels of positive end-expiratory pressure (matched to IAP) and small-volume resuscitation with hyperoncotic albumin 20%, followed by fluid removal with furosemide (Lasix®) or ultrafiltration. This approach was associated with negative fluid balance, lower intra-abdominal pressure, lower extravascular lung water index, fewer days of mechanical ventilation and ICU admission, as well as lower 28 day-mortality [43].
THE PARADIGM SHIFT
A paradigm shift in fluid management is occurring; recognition of increased morbidity and mortality related to fluid overload has led modern strategies to place more emphasis on the risks rather than benefits of IV fluid administration.
In healthy individuals, only 25% of a crystalloid bolus remains intravascular after 3 hours; 75% is leaked into the interstitial space. Experimental models of sepsis demonstrate almost complete loss of IV fluids to the interstitium, resulting in pleural effusion, ascites, organ oedema, and impeding organ function. During critical illness, the cytokine storm and ensuing capillary leak results in the passage of intravascular free water, electrolytes, proteins, and albumin into the interstitium. It therefore follows that, except where specifically indicated, indiscriminate and aggressive IV fluid administration in critically ill patients is often unnecessary and may be harmful.
The magnitude of positive fluid balance may be considered a biomarker of critical illness. Patients successfully resuscitated from shock usually achieve pro- and anti-inflammatory mediator homeostasis within 3 days; subsequent haemodynamic stabilisation and restoration of plasma oncotic pressure allows diuresis and mobilisation of extravascular fluid to achieve a negative fluid balance. The return of cytokine homeostasis allows repair of the microcirculation and cessation of capillary leak.
In contrast, patients with a persistent systemic inflammatory response fail to reduce transcapillary albumin leakage and accumulate increasingly positive net fluid balances - a state known as Global Increased Permeability Syndrome (GIPS) [54, 55]. Administration of IV fluids in patients with GIPS further increases the pressure in the 4 main compartments of the body: the head, chest, abdomen, and limbs, with the decreased flow gradients in distal organs compromising organ function. Not only should these patients not be given IV fluids, but active steps should also be taken to eliminate excess fluids (LGFR). The ROSE acronym neatly summarizes the dynamic phases of fluid therapy: Resuscitation, Optimization, Stabilization, and Evacuation [3] (Table 6).
TABLE 6
ROSE diagram illustrating the dynamic phases during fluid therapy (adapted from Malbrain et al. with permission [3])
Fluid removal can be attempted via loop-diuretics, or a diuretic combination therapy, or even slow continuous ultrafiltration (SCUF), with the aim to restore homeostasis while avoiding deleterious effects such as electrolyte imbalances, metabolic alkalosis and acute renal injury. Comorbidities should also be considered as conditions such as renal or heart disease may limit the response to deresuscitation; development of dynamic prediction models based on daily measures of fluid responsiveness can help identify patients benefiting from diuretics and/or SCUF. The use of hypertonic solutions in combination with diuretics only makes physiological sense in patients with congestive heart failure, whereas in other critically ill patients with normal cardiac function this may have more adverse effects [118].
CONCLUSIONS
Excessive intravenous fluid administration is associated with increased morbidity and mortality. IV fluids should be considered as drugs and only administered where specifically indicated. Critically ill patients will benefit from precise fluid management strategies individualised for their condition – it is not a ‘one size fits all’ situation, and patients should not be uniformly fluid-resuscitated to the point at which they are no longer fluid-responsive.
Several techniques are available to assess fluid status and monitor progress, with bedside ultrasound showing a great deal of promise as an inexpensive, non-invasive, and accessible tool. Fluid balance is a dynamic process and should be actively managed as such. It is important to identify the patients who will benefit from fluid resuscitation as well as those who should be de-resuscitated.







