Ceftazidime-avibactam (Zavicefta®) is an intravenously administered combination of the third-generation cephalosporin and the novel β-lactamase inhibitor [1]. Hypersensitivity reactions to β-lactam antibiotics can be classified as immediate or non-immediate according to the latency period between the last drug administration to symptoms and clinical presentation [2].
In vitro tests for the diagnosis of penicillin allergy provide for the dosage of serum IgE for penicilloyl G and V, ampicillin and amoxicillin. However, this diagnostic procedure suffers the disadvantage linked to the reduction of specific IgE serum levels for a certain drug, producing false negative results, if the time between the reaction and the execution of the test exceeds 1 year [3]. Despite its great usefulness to detect allergen sIgE, its sensitivity is variable and not optimal (0–50%) and it could depend on the severity of the clinical symptoms [4]. Therefore, these tests are less sensitive than skin tests but represent a useful complement of the diagnostic workup.
We have previously developed a flow cytometry-based assay to assess the antigen specific B and T cell proliferation in vitro [5], allowing us to detect both immediate and delayed hypersensitivity reactions, respectively.
A 63-year-old female affected by diffuse subarachnoid haemorrhage due to rupture of the anterior communicating artery’s saccular aneurysm, dyslipidaemia on Atorvastatin treatment, allergy to ceftriaxone, penicillin V and nickel, chronic respiratory failure.
She reported appearance of widespread urticaria, which developed after the administration of Zavicefta (ceftazidime-avibactam), i.e. the reason why an allergy consultation was requested.
In order not to interrupt the antibiotic therapy, it was decided to start a rapid desensitization protocol according to Bavbek et al. [6], originally developed for chemotherapeutic agents and irreplaceable drugs. Towards the end of the procedure, reappearance of erythematous and itchy skin rashes occurred on the lower limbs, which led to the interruption of the antibiotic infusion and administration of trimeton and flebocortid 200 mg. The patient did not develop angioedema and she did not experience any adverse respiratory, cardiac or gastrointestinal symptoms.
Resolution of the hypersensitivity symptoms occurred within an hour of treatment and vital signs remained stable. The urticarial reaction during the 12-step protocol represented further evidence of immediate hypersensitivity of the drug.
The case was subsequently investigated using the flow cytometry-based proliferation assay, so the lymphocytes of the patient were incubated for 5 days with three different Zavicefta concentrations: dilution 1/10 (XD, excess dose), 1/100 (TD, therapeutic dose) and 1/1000 (LD, low dose).
The XD was cytotoxic, inducing high lymphocyte-cell death. Considering the B cells, the test provided a ratio of almost 4 for the TD and was thereby considered positive. To confirm this latter result, we performed the same test with Starcef (ceftazidime), i.e. cephalosporin only without β-lactamase inhibitor. T cells, however, did not proliferate in response to either drug.
Comparing both results, it is possible to conclude that the hypersensitivity reaction, manifested by the patient, was most certainly due to the third-generation cephalosporin, excluding the role played by the β-lactamase inhibitor. Further proof was given by the positive results of Rast β-lactams. T lymphocytes have also been studied, without producing significant results.
The antibiotic was administered in subsequent 15-minute intervals for a total of 12 progressively doses with continuous monitoring. According to the literature, this interval time is the safest to avoid major adverse reactions in the desensitization procedure [7]. Indeed, in in-vitro models of basophil desensitization, it has been demonstrated that when human basophils are incubated with suboptimal doses of the allergen, they reach the minimum responsiveness in a time interval between 15 and 30 min [8].
This is not the first reported case in the literature to describe a type I hypersensitivity reaction with rapid IV induction of tolerance to ceftazidime/avibactam [9], but it is the first in which we tried to investigate which component of Zavicefta triggered the hypersensitivity and we showed it not only according to the patient’s clinical scenario, but also through a flow cytometry-based proliferation assay.
Analysing the results of the proliferation assay, we saw how the B lymphocytes proliferated at a higher rate, compared to T lymphocytes, suggesting an immediate hypersensitivity reaction, most likely due to ceftazidime. Among the limits of this test, there was the lack of possibility to study the effect of avibactam individually, which could increase or reduce the hypersensitivity.
The 12-step protocol was structured to reach the final dose of the drug equal to 2 g. As stated by the Zavicefta leaflet, the best and safest therapeutic concentration is 20 mg/ml of ceftazidime, sufficient for most scenarios, in a tolerability range of 8–40 mg/ml, therefore suitable with the statement.
The protocol of Coop et al. [9] reached a concentration of 50 mg/ml, reaching a faster desensitization protocol, but over the therapeutic concentration range. In fact, the total infusion time was 187 min, compared to ours of 252 min. Then, using the final solution (Solution B – Table 1, Figure 1), we did not exceed the infusion rate of 50 ml/h, considering that Zavicefta is administered by intravenous infusion over 120 min in an appropriate infusion volume of 100 ml.
Figure 1
Flow cytometry-based proliferation assay. Freshly isolated peripheral blood mononuclear cells of the patient are stained for 5 min with carboxyfluorescein succinimidyl ester (CFSE), washed and incubated for 5 days with 3 different 10-fold drug dilutions, one of which is a “therapeutic concentration”, calculated on the distribution volume of the drugs. In the case, the steady-state volumes of distribution of ceftazidime and avibactam are comparable about 17 and 22L. Cells incubated with phytohemagglutinin A (PHA) or no stimulus were used as positive and negative controls, respectively. All cultures were performed in triplicates. At the end of the 5-day incubation, cells were harvested, washed and stained with fluorochrome-coupled anti-CD3 and anti-CD19 antibodies to distinguish between T and B cells, respectively, then analysed using a flow cytometer. Using this technique, we can measure the percentage of B and T cells that have proliferated: indeed, proliferating cells have a reduced CFSE intensity as compared to resting cells. The test is deemed positive if the B cell or T cell proliferation rate of any of the drug concentrations tested equals or exceeds 2, as compared to the negative control
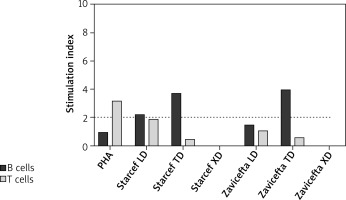
Table 1
Ceftazidime/avibactam 12-step desensitization protocol. Four solutions were prepared: Solution A – 2 g ceftazidime/0.5 g avibactam vial to be diluted in 10 ml of sterile water for injections (2 g/ml); Solution B – solution A (volume to withdraw from a reconstituted vial – approximately 12 ml) in 88 ml of the physiological solution (20 mg/ml); Solution C 1/10 – 5 ml of solution B in 45 ml of the physiological solution (2 mg/ml); Solution D 1/100 –5 ml solution 1/10 in 45 ml of the physiological solution (0.2 mg/ml). The target dose of 2 g was successfully achieved within a 252 min’ interval. After this step, the target dose of Zavicefta was administered every 8 h
In conclusion, this case report highlights: I) the possibility of hypersensitivity reactions to new generation β-lactam antibiotics, so the need to implement the research and diagnostics both in vivo and in vitro; II) the use of in vitro CFSE lymphocyte proliferation test to demonstrate also immediate reactions, not only for the delayed ones, especially in conditions where it is not possible to make diagnosis in vivo; III) the desensitization protocol used is safe and effective, demonstrating a successful rapid induction of tolerance for the ceftazidime/avibactam antibiotic, concluding the cycle of antibiotic therapy without further adverse reactions.








