Introduction
Cutaneous malignant melanoma (MM) is still a challenging issue in the field of dermato-oncology alike surgical oncology and clinical oncology [1]. It is estimated that the incidence of this lethal skin cancer has rapidly increased in the last 5 decades. The middle European incidence ratio ranged from the number of 3 new diagnosed cases per 100,000 inhabitants in the early 1970’ to the ratio of 9 new diagnosed cases per 100,000 inhabitants in the beginning of the 21st century [2]. Cutaneous MM causes around 55,500 deaths per year worldwide and belongs to the highest mortality cancers, accounting for approximately 75% of deaths from all skin cancers [3]. An emerging issue among MM patients is late-stage diagnosis, as well as its biological predisposition to rapid growth and difficulties with obtaining control progression stage [4]. That is why in recent years, the treatment of MM in the advanced clinical stage has become a strategic and emerging issue. Currently, many interesting treatment options have been proposed for this specific group. The most currently evaluated clinical strategies include the development of immunotherapy, the use of targeted agents like BRAF and MEK inhibitors or novel products of oncolytic viral therapy [5, 6]. Moreover, among the significant group of patients, excluding those with distant metastases, surgery supported or replaced by radiotherapy, isolated perfusion hyperthermia and electrochemotherapy (ECT) are currently the first choice treatment. From the above-mentioned methods, the ECT has become an increasingly interesting direction in the development of local treatment of the advanced superficial tumours and/or their satellite and in-transit metastases [7, 8].
Aim
The aim of this study was to analyse the clinical effectiveness of the ECT in the treatment of advanced metastatic malignant melanoma in the group of patients hospitalized in the second Department of Surgical Oncology of the Wroclaw Comprehensive Cancer Centre, Poland.
Material and methods
Patients/Study group
There were 4 men and 1 woman (mean age: 70.2, range: 60–76) diagnosed with melanoma at varying grades of advancement of MM who have undergone the ECT (Table 1). Three patients had T2b primary tumour stage (dimension: 1.5–1.8 cm, with ulceration); one had T3 and one T4b (dimension: 10.5 cm). All patients before the ECT had primary tumours resected which were located in four out of five patients within the limbs and in one case within the trunk. Patient 3 presented a distant metastasis to the retroperitoneal space which was excised before the ECT and, as in patient 2, was diagnosed with BRAF positive melanoma. It is worth noting that patient 5 was successfully treated for sigmoid cancer (pT2N0M0) in 2015. Patient 1 was qualified to the ECT after almost 30 years from the diagnosis. In the rest of the patients the mean interval between diagnosis and the ECT cycle was 2 years and 3 months (6 months–4 years, 11 months).
Table 1
Patients’ characteristics
Patients were qualified for the ECT in different clinical situations, however, all of them already had a surgical intervention for the primary MM. In total, immunotherapy was administered in three patients, of whom two underwent radiotherapy (Table 2). All but one patient already had a lymphadenectomy which in patient 2 was performed for the second time. Moreover, patients 1 and 4 had multiple reoperations (9 and 7, respectively) of the in-transit metastases due to locoregionally relapsed disease and patient 5 underwent one such surgical procedure.
Procedure and clinical evaluation
The ECT was performed according to the European Standard Operating Procedures of Electrochemotherapy (ESOPE) [9]. Bleomycin was administered intravenously in patients under general endotracheal anaesthesia and in supine position before the procedure. The dosage was measured depending on the body surface. The electroporation procedure has started 8 min after intravenous injection of bleomycin. A square wave electroporator, Cliniporator model EPS02 (IGEA, Carpi, Italy), was used to deliver the electric pulses. Linear (N-20-4B) or hexagonal (N-20-HG and N-30-HG) electrodes measuring 20–30 mm in length, chosen by the clinician before the ECT, were used on all clinically evident in-transit metastases. Pulses, also chosen based on the clinician’s experience, were delivered in two ways: 1) sequences consisting of 4 high voltage pulses with 730 V amplitude and 100 µs duration; 2) sequences consisting of 8 high voltage pulses with 400 V amplitude and 100 µs duration. Detailed data about the whole procedures including time, duration of the pulses, measurement of the applied voltage and current were exported from the electroporator and then analysed.
The treatment response, evaluated 4 weeks after the procedure, was the mean of the response for each lesion based on Response Evaluation Criteria in Solid Tumours [10]. The site of the treatment was photographed on the day of the procedure, 1 month and 2 months later.
Results
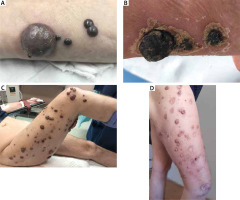
Since the introduction of the ECT in the Wroclaw Comprehensive Cancer Centre, 6 procedures were performed in 5 patients. The dose of bleomycin varied from 26k to 30k UI (Table 3). In case of patient 4 during the first cycle of ECT the linear electrode was used. The rest of the patients were treated with hexagonal electrodes. In some patients, the accurate assessment of the number of lesions was impossible due to their fusion (Figure 1), therefore the description of the procedures included the number of electrode positions. In total, 377 (18–187) electrode placements have been performed to treat approximately 200 nodules. The length of the procedure, calculated from the beginning of the first to the end of the last electroporation, was closely related to the regional relapse of melanoma and lasted on average 10 min 41 s.
Table 3
ECT procedural details
Figure 1
Comparison of treatment effects of well-separated and overlapping metastases in 2 patients. A – Patient 3 on the day of the procedure; B – patient 3 2 months after the procedure; C – patient 5 on the day of the procedure; D – patient 52 months after the procedure
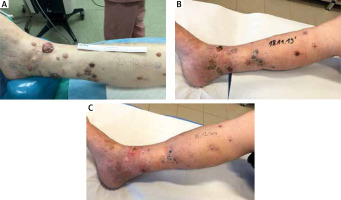
After 5 out of 6 ECT procedures, partial treatment response has been achieved one month after the ECT. In patient 1, all of the lesions were reduced and 4 months after the procedure four of them were visible. Partial responses were also observed in patient 2, 3 and 5, however, in patient 3 three and in patient 5 one new, untreated lesion has appeared. In patient 4 after the first cycle of the ECT the treated nodules progressed. Moreover, one new lesion was found after the first ECT. In this case, first-line immunotherapy administered was ineffective and the second cycle of the ECT preceded by an amputation of the hallux was proposed. After the repeated cycle, partial response has been observed (Figure 2). In all but one patient, first or subsequent resections of the cutaneous metastasis were undertaken after the ECT. In total, reoperations were performed 5, 1, 1, 4 times in patients 1, 2, 3, and 4, respectively.
Figure 2
Patient with multiple in-transit metastases on the right foot and leg. A – On the day the second ECT was performed; B – 1 month follow-up; C – 2 months follow-up
Patients were followed until 30 November 2020. One patient died 1 year, 7 months and 3 days after the ECT despite the notable treatment response. The cause of death was a thromboembolic phenomenon correlated with aggravating clinical condition as a consequence of the course of MM. The mean time from the procedure to that date in the rest of the group was 1 year, 6 months and 6 days. In patients 2 and 4, long-term disease stabilization was achieved. Although the assessed response for the ECT was partial, the subsequent removal of the lesions in these patients did not allow for future recurrence. In long-term observation in patient 3 MM progressed and the multiple distant metastases (among others to the lymph nodes, kidneys and orbit) were diagnosed 4 months after the ECT. Patient 5 did not come for the last follow-up visit in November 2020 (5 months after the ECT) and a wider evaluation of his clinical condition was not possible.
Of note, an interesting correlation was observed during the study. Basal Cell Carcinoma was diagnosed and surgically excised in patients 1–3. Moreover, in patient 2 there was a relapse of BCC. In total, BCC was diagnosed twice before the melanoma and twice after. This observation is in agreement with the literature data about BCC as a risk factor for MM and an increased risk of subsequent skin cancer after the MM diagnosis [11, 12].
Discussion
Electrochemotherapy is a relatively novel and cost-efficient method of treatment of metastases in patients with advanced cutaneous melanoma [13, 14]. Since 2006, when the ESOPE established standardized details of the ECT procedure [10], the increasing popularity of the use of this method has been observed in cases of lack of response to treatment with other methods and in non-resectable lesions from MM [15]. Furthermore, high efficacy and excellent safety profile was proved for this method which resulted in the introduction of the ECT in several international guidelines [16–18]. The authors are aware of a small study sample size, which is a relevant limitation of this article, especially in view of above-mentioned facts. However, despite the increasing role of ECT in the treatment of advanced MM, access to this method in Poland remains limited and it is available to patients only in a few specialized centres in the whole country.
Our paper presents the effects of treatment of patients with multiple lesions, involving whole limbs as well as individual lesions. In both situations it was possible to achieve the particular response with long-lasting stabilization of the disease, and even to prevent relapse during the course of the study. Moreover, as the treatment is well tolerated in case of progression it is possible to retreat the patient with another cycle [19].
Highly positive outcomes after the ECT cycle are consecutively repeated in recent studies. In a literature review published in 2018 and consisting of analysis of 11 articles from 2006–2017, the results of the ECT treatment of cutaneous MM from different centres were shown [20]. In total, the review gathered 502 patients with 2050 lesions and a mean overall response was 74% (complete response (CR) and partial response (PR) were 40% and 34%, respectively). In 2018, Al-Hadithy et al. reported the results of the prospective cohort study on superficial cancers of 48 patients, of whom 26 were diagnosed with melanoma [21]. In this group, in 24 cases good or partial response was achieved (92%). Very good clinical efficacy of the ECT was presented by the cancer centres collaborating within the International Network for Sharing Practices on Electrochemotherapy (INSPECT) [22]. Two hundred and eighty-three melanoma patients with 932 cutaneous lesions were included in the study. The OR and PR were 82% and 64%, respectively. Unfortunately, however, regardless of the encouraging clinical effectiveness of electroporation, some research groups reported lack of impact on overall survival [23, 24].
An important aspect for patients who suffer from advanced cutaneous melanoma is the impact of the skin-related symptoms on their quality of life [25]. Hence, in addition to the cosmetic effect, it is worth paying attention to the fear of recurrence and distress from the widespread of the nodules within the skin as well as ulceration and pain of the advanced cutaneous metastases [26]. Worth noting is that oncological treatment itself affects the patients’ life and may be refused due to possible complications. Multiple resections or limb amputations are especially harmful and negatively perceived by patients who suffer from MM [27]. Thus, less invasive therapeutic solutions may improve both the patient’s well-being and compliance.
Conclusions
In recent years the number of ECT procedures has been increasing systematically as well as the role in the treatment of cutaneous MM. An analysis presented in this paper confirmed that the ECT is an effective therapeutic approach in non-resectable and treatment-resistant in-transit metastases. Within the study period, except the positive response after the procedures, the stabilization and control over the relapse of MM was observed. Furthermore, what is especially important for the patients, visible reduction in the lesions and improvement of skin appearance positively influencing their quality of life was obtained. Although ECT is still under active investigation and further studies are necessary to consolidate the scientific evidence of utility of this form of treatment, it seems that properly selected groups of patients may get particular benefits even in the terminal stage of MM.








