Introduction
It has been established that in psoriasis, a systemic immune-mediated inflammatory disease of unknown origin, abnormal proliferation of keratinocytes, neoangiogenesis and activation of the immune system are the three fundamental processes responsible for the development of psoriatic skin manifestations. The presence and interactions of CD8+ T cells and neutrophils in the psoriatic epidermis as well as dendritic cells (DCs) and CD4+ T cells in the psoriatic dermis mediate the disruption of the immune tolerance. The imbalance between the regulatory T (Treg) cells, essential for maintaining immune homeostasis, and the effector T cells (i.e. Th1, Th17, Th22), accountable for activation of the immune response, has been observed not only in the skin of psoriatic patients but also in their blood, which appears to confirm the systemic nature of psoriasis [1–3]. Therefore, in order to shed more light on the molecular interactions in psoriasis pathogenesis, the immune mechanisms that fail to control both regulatory and effector T cells need to be thoroughly investigated and the role of such molecules as programmed death 1 (PD-1), neuropilin 1 (NRP-1) and human leukocyte antigen-G (HLA-G) should be carefully analysed.
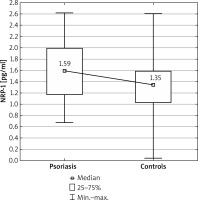
Figure 1
Comparison of neuropilin 1 (NRP-1) concentrations between the psoriatic patients and control group
In order to activate the T cells, which will subsequently trigger a cascade of immune responses, at least two signals are required. The first one involves interaction between the T cell receptor (TCR) and major histocompatibility complex (MHC) on the antigen-presenting cell (APC), while the other signal may be either stimulatory or inhibitory. Programmed death 1, a checkpoint inhibitor expressed on the T cells, B cells and macrophages, is engaged in the latter. However, any disturbance in the interaction between PD-1 and its ligands, i.e. PDL1 or PDL2, mainly expressed on the APC, as observed for example during anti-PD-1 treatment, will increase the T cell activation and pro-inflammatory cytokine production. Besides membrane-bound PD-1, a soluble form (sPD-1) which lacks the transmembrane domain is encoded. Soluble PD-1 is able to block the PD-1/PDL-1 pathway and promotes T cell responses toward pro-inflammatory Th1/Th17 [4, 5]. All this will be manifested in autoinflammatory or autoimmune diseases, e.g. in psoriasis.
Human leukocyte antigen-G (HLA-G) is another checkpoint molecule which plays a key role as an anti-inflammatory and tolerogenic protein [6]. It induces apoptosis in the T and NK cells and inhibits cytotoxic T-cell activity, therefore, all this can be considered a negative feedback signal that downregulates lymphoid reactions and inflammatory processes. Human leukocyte antigen-G is a non-classical major histocompatibility complex (MHC) class Ib molecule which consists of seven isoforms, i.e. membrane-bound (HLA-G1 to HLA-G4) and soluble (HLA-G5 to HLA-G7). The soluble HLA-G (sHLA-G) is mainly produced and secreted by the immune cells, including monocytes/macrophages and myeloid/plasmacytoid DCs [7]. Higher plasma levels of sHLA-G have been reported in various types of cancer and viral infections [6, 8–10]. Thus, it may be speculated that decreased HLA-G levels in psoriasis could favour the development of the disease.
Neuropilin-1 is a co-receptor for vascular endothelial growth factor (VEGF), a molecule responsible for the pro-angiogenic effect, and it also plays a role in T cell and DC immunoregulation [11, 12]. In psoriasis, elongated, tortuous capillaries in the dermis are the features of angiogenesis and progression of the disease [13]. NRP-1 is expressed on Tregs, myeloid cells, including DCs, as well as on neurons and blood vessel cells [11]. NRP-1 has been found to be involved in VEGF-mediated inhibition of DC maturation, an important immunosuppressive mechanism [11]. Since soluble NRP-1 (sNRP-1) isoforms lack the MAM/c, transmembrane and cytoplasmic domains, they can bind NRP-1 ligands, but are unable to transduce signals, and thus they may serve as decoy receptors/natural inhibitors to sequester the NRP-1 ligands [14, 15]. This will result in chronic activation of DCs and undesired immune response, e.g. in psoriasis.
Aim
In order to better understand disturbed immune tolerance in psoriasis, this study investigates the levels of sNRP-1, sPD-1 and sHLA-G in the circulation of psoriatic patients.
Material and Methods
Studied group
The study comprised 57 male psoriatic patients hospitalized in the Department of Dermatology, Venereology and Pediatric Dermatology, Medical University of Lublin, Poland and 29 male healthy controls. Fifteen patients (26.32%) suffering from psoriatic arthritis (PsA) met the Classification of Psoriatic Arthritis (CASPAR) criteria. After the demographic data and medical history had been gathered, the clinical examination of each studied patient was performed and blood specimens were collected.
Assessment of psoriasis severity
In all the studied patients the severity of psoriatic skin changes was assessed using the Psoriasis Area and Severity Index (PASI), body surface area (BSA), Physician Global Assessment (PGA) and Dermatology Life Quality Index (DLQI) scores.
Assessment of concentrations of selected immune tolerance mediators in plasma of studied psoriatic patients and controls
The blood specimens previously collected from the psoriatic patients and controls were centrifuged for 15 min at 1000 × g. Then, the plasma samples were subdivided into small aliquots and stored at –80°C to be subsequently tested for the selected immune tolerance mediators’ concentrations. All the concentrations were determined in compliance with the manufacturer’s instructions and using the Human Programmed cell death protein 1 ELISA Kit (EIAab), Human neuropilin-1 (R&D Systems) and sHLA-G ELISA (BIOVendor).
The study was approved by the Local Ethic Committee at the Medical University of Lublin (KE-0254/81/2015). Informed consent was obtained from all the participants.
The study was supported by grant No. 164 of the Medical University of Lublin.
Statistical analysis
The data were statistically analyzed using Statistica 10.0 PL (StatSoft Inc., USA). The median values with the (min.–max.) range, and lower and upper quartiles, were calculated for the continuous variables, whereas the absolute (n) and relative numbers (%) were calculated for the categorical variables. The Mann-Whitney U test was used to compare age and the selected immune tolerance mediators plasma concentrations between psoriasis patients and the control group as well as between psoriatic patients with and without arthritis. Spearman’s correlation coefficient was used to assess correlations between the selected immune tolerance mediators’ plasma concentrations and clinical data in the psoriatic patients.
The value p < 0.05 was considered to indicate a significant difference.
Results
Socio-demographic characteristics and clinical data of the studied psoriatic patients are presented in Table 1.
Table 1
Clinical data of psoriatic patients (N = 57)
| Clinical data | Min. | Max. | Median | Lower quartile | Upper quartile |
|---|---|---|---|---|---|
| Age [years] | 21 | 76 | 46 | 35 | 58 |
| Duration of the disease [years] | 1 | 55 | 19 | 10 | 29 |
| Age of disease onset [years] | 1 | 71 | 21 | 17 | 35 |
| PASI | 3 | 43 | 12 | 8 | 18 |
| PGA | 2 | 5 | 3 | 3 | 4 |
| BSA | 2 | 75 | 15 | 10 | 35 |
| DLQI | 1 | 30 | 11 | 8 | 20 |
The studied psoriatic patients’ median age was 46 years and did not significantly differ with respect to the control group’s age (p = 0.516). Duration of psoriasis ranged from 1 to 55 years with the median value of 19 years. Twenty (35.09%) patients had positive psoriatic family history.
The PASI value in the studied group ranged from 3 to 43; the median value was 12. The BSA value was in the range of 2–75%, median value 15%. The median value of the PGA was 3.
Comparing plasma concentrations of selected immune tolerance mediators between the studied psoriatic patients and controls
The serum concentrations of NRP-1 in the studied psoriatic patients was significantly higher than in the control group (p = 0.010) (Figure 1). However, no significant differences were found in the PD-1 and HLA-G concentrations between the psoriatic patients and the control group (p = 0.094 and p = 0.482, respectively) (Table 2).
Table 2
Comparisons of selected immune tolerance mediators’ plasma concentrations between psoriatic patients and control group
Comparing plasma concentrations of selected immune tolerance mediators between the psoriatic patients with and without arthritis
No significant differences were found in the sPD-1, sNRP-1 and sHLA-G concentrations between the psoriatic patients with and without arthritis (p > 0.05) (Table 3).
Analysis of correlations between selected immune tolerance mediators’ concentrations and clinical data in the studied psoriatic patients
Table 3
Comparisons of selected immune tolerance mediators’ plasma concentrations between psoriatic patients with and without arthritis
No significant correlations were found between the concentrations of the selected immune tolerance mediators and psoriasis duration, psoriatic arthritis duration, or psoriasis severity expressed by PASI, BSA, and PGA (p > 0.05) (Table 4).
Table 4
Correlation coefficients between selected immune tolerance mediators’ plasma concentrations and clinical data in psoriatic patients
Discussion
The mechanisms of development, function and stability of the immune system, which are known to rely on the cell-to-cell contact and/or participation of cytokines, have been confirmed to play a pivotal role in both human physiology and pathology [16]. Since it has been shown that the Treg cells are capable of suppressing both activation and functioning of the Th cells, B cells and DCs [17], their preventive role in the pathogenesis of autoimmune and autoinflammatory diseases is unquestionable. In psoriasis, an autoinflammatory disease, the down-regulation and defective functioning of Tregs have also been confirmed both in the peripheral blood and in skin lesions [3]. Thus, the long contact between Tregs and DCs, an essential mechanism of immune tolerance, in psoriasis may be compromised, leading to autoimmunity. There are a few cell-surface molecules responsible for induction and maintenance of immune tolerance, i.e. NRP-1, PD-1, and HLA-G, but their possible role in psoriasis still awaits further clarification.
It should be noted that the cytokines involved in triggering immune tolerance produced by Tregs, i.e. transforming growth factor β (TGF-β) and interleukin 2 (IL-2), control the expression of PD-1 and NRP-1 on Tregs, as well as Treg cells’ development into follicular regulatory T (Tfr) cells [18]. Transforming growth factor β promotes conversion of CD4+ T cells into Treg cells, while a deficit of the cytokine favours the conversion of Treg cells into Th1 and Th17 cells [18, 19]. IL-2 induces Foxp3 (Forkhead box p3), a known transcription factor which controls Treg development and function. PD-1 is known to be expressed on the surface of activated lymphocytes and the interaction of PD-1 with its ligand PD-L1 leads to suppression of T cell receptor signalling and generation of Treg cells from naïve CD4+ T cells [1, 18]. The soluble form of PD-1, however, acts as a decoy receptor able to bind and neutralize PD-1/PDL1 [4, 5]. In our recent studies we have found that both the expression of PDCD1 mRNA in peripheral blood mononuclear cells [20] and the expression of PD-1 protein on CD4+ and CD8+ T cells were significantly decreased in psoriatic patients both with and without arthritis [21]. Thus, the compromised PD-1 function on CD4+ and CD8+ T cells might indicate inappropriate activation status and suggest dysregulation of the immune suppression mechanisms, which may lead to abnormal, persistent T cell activation and cytokine production in psoriasis. Since no significant difference has been found in the plasma concentration of sPD-1 between the psoriatic patients and the controls, it seems that this study may provide further evidence confirming the presence of some disturbed feedback within the PD-1/PDL1 pathway.
Interestingly, Liu et al. [4] reported that their rheumatoid arthritis (RA) patients presented high levels of serum and synovial concentrations of sPD-1, which is known to be able to neutralize PD-1 on T cells [4]. This may well explain the contribution of sPD-1 to the increased immune response observed in RA.
NRP-1 and sNRP-1 act in the opposite way to one another in angiogenesis and the immune response, two fundamental processes in psoriasis pathogenesis. sNRP-1 is a decoy receptor able to bind its ligand and prevent long-term interactions between Tregs and immature DCs, thereby promoting immunity [22].
In our present study, the circulating sNRP-1 was significantly higher in psoriatic patients in comparison to the controls, whereas in one of our earlier studies, the expression of NRP-1 mRNA in peripheral blood mononuclear cells (PBMCs) was significantly downregulated in psoriatic patients, both with and without arthritis [21]. Therefore, the increased sNRP-1 concentrations observed in our psoriatic patients could be regarded as a negative feedback mechanism in the process of neovascularization, on the one hand, but, on the other hand, they could also be seen as an immune tolerance compromising factor.
Soluble HLA-G is a molecule capable of suppressing the immune response and promoting the development of Tregs. Borghi et al. [23] observed significantly lower plasma levels of sHLA-G in psoriatic patients than in healthy volunteers. However, in our study no difference in the plasma levels of sHLA-G in the psoriatic patients in comparison to the control group was found. Since the sHLA-G level in our psoriatic patients was not different from the sHLA-G level in the controls, the molecule’s immune suppressive and protective functions against inflammation might be insufficient and unable to prevent the progress of psoriasis. Thus, the lack of difference in sHLA-G concentration between psoriatic patients and healthy volunteers might suggest an insufficient mechanism which could limit immune responses and suppress pathologic immunity. Interestingly, in our previous study we did not find any difference in the mRNA HLA-G expression between the psoriatic patients and controls [20]. Both Aractingi et al. [24] and Cardili et al. [25] observed enhanced HLA-G expression within the psoriatic lesions, which may be suggestive of some modulating role of HLA-G+ T cells at the site of inflammation. Sweeney and Kirby [26] suggested that in psoriasis HLA-G might promote the development of Tregs and prevent tissue destruction.
Verbruggen et al. [27] in their RA patients observed lower plasma levels of sHLA-G in comparison to the healthy individuals. Thus, the low levels of sHLA-G observed in those patients could indicate inability of the immune system to suppress the development of self-reactive cells, leading to the development of an autoimmune disease. Veit et al. [28], however, observed high sHLA-G levels in patients with continuous anti-RA treatment, which could reflect an attempt of the immune system to counterbalance the autoimmune process. The same authors [28] also noted that the circulating sHLA-G levels were increased in the late stage of RA.
Conclusions
Analysis of the circulating sPD-1, sNRP-1 and sHLA-G provides evidence confirming their compromising effect on building immune tolerance in psoriatic patients. However, it goes without saying that further elucidation of the role of these molecules in the modulation of immune tolerance will contribute to better understanding of the complex molecular mechanisms involved, inter alia, in psoriasis.








