Introduction
Posttranslational modifications (PTMs) of proteins are seen as a strategy of individual cells to rapidly respond to environmental changes [1]. These modifications can occur spontaneously or by enzymatic modifications of certain amino acids within the protein. They participate in a broad spectrum of biological processes (gene expression, signal transduction, folding of proteins, cell division, cell differentiation, apoptosis, antigen recognition and immune response), and the abnormal status of PTMs is frequently implicated in various autoimmune diseases [2-6]. Common PTMs are phosphorylation, citrullination, acetylation, glycosylation, methylation, deamination, carbamylation, and ubiquitination, but among them, citrullination (deimination) has been the most studied in recent years [7-10]. This modification involves the conversion of arginine to a citrul- line residue with the participation of the enzymes peptidylarginine deiminases (PADs) [11]. The increased interest is due to the fact that more than 80% of patients with rheumatoid arthritis (RA) have elevated levels of anti-citrullinated protein antibodies (ACPAs), and these antibodies appear many years before the clinical onset of the disease [12-14]. Therefore, they can be used simultaneously for diagnostic and prognostic purposes, as well as for monitoring of the ongoing treatment [15-17]. ACPAs were included as a specific biomarker in the 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) RA classification criteria [18].
Since the ACPAs isolated from RA patients cross-react with various citrullinated proteins (fibrinogen, collagen type II, α-enolase, vimentin, fibronectin, β-actin, apolipoprotein) as well as with proteins that have undergone other posttranslational modifications (carbamylation and acetylation), it is increasingly believed that the citrullination process itself, rather than a specific citrullinated antigen, plays a role in the pathogenesis of RA [13, 19, 20]. Thus, the role of ACPAs in RA development is not yet fully recognized. Taking into account that ACPAs can be detected several years before the disease onset, it is not clear which factors are responsible for their appearance. Possible concepts for the involvement of ACPAs in the pathogenesis of RA have been discussed by Volkov et al. [21].
The development of autoreactive B cells and ACPAs is T cell dependent. Inflammation of the synovial membrane and destruction of the joint cartilage are key elements in the pathogenesis of RA. Therefore, autoantigens are required to initiate an immune response. As a major component of articular cartilage, collagen type II (CII) and its post-translationally modified forms are suitable candidate autoantigens in the RA pathogenesis. Citrullinated at position 315 CII311-325 peptide was reported as a novel human leukocyte antigen (HLA) restricted T cell epitope (HLA-DRB1*10:01) [22]. The authors demonstrated that citrullinated CII311-325 peptide (but not unmodified CII311-325) can bind to HLA-DRB1*10:01, can activate CD4+ T cells, and can induce production of proinflammatory cytokines [22]. They did not show whether this epitope is recognized by ACPAs, but other papers reported that ACPAs recognize the “Cit-Gly” motif, which is actually present in this epitope [20, 23]. Nevertheless, the mechanisms of interaction between B cells and T cells, the production of ACPAs, and what initiates the onset of disease remain unknown.
The well-known major immunodominant T cell epitope within CII (CII259-273) is a target for other posttranslational modifications but not citrullination. We have previously shown that by mimicking this epitope, the peptide L-ASNase67-81 (part of bacterial L-asparaginase) can activate HLA-DRB1*04:01 restricted CD4+ T cells and induce secretion of interleukin (IL)-2, IL-17A/F and interferon γ (IFN-γ) in patients with early RA (ERA) [24]. L-asparaginase (EC 3.5.1.1 or EC 3.5.1.38) is a homotetrameric enzyme that catalyzes the hydrolysis of asparagine to aspartate (or glutamine to glutamate) and ammonia (Fig. 1) [25].
Fig. 1
Schematic illustration of the dual activities of bacterial L-asparaginase. (-NH2) – amino group, (-OH) – hydroxyl group, O – oxygen, H2O – water, NH3 – ammonia
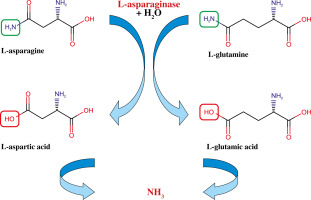
This activity of the enzyme is mainly used for treatment of acute lymphoblastic leukemia (ALL), because the neoplastic cells cannot synthesize asparagine, and to survive and proliferate, they need an exogenous source of asparagine [26-28]. Administration of L-asparaginase leads to hydrolysis of the circulating extracellular asparagine causing starvation and death of the tumor cells. As an extension of our previous study, we wanted to test whether the bacterial L-asparaginase is able to modify the two T-cell epitopes within CII (CII259-273 and CII311-325) as they contain asparagine or glutamine. Therefore, we examined the enzyme’s ability to hydrolyze asparagine/glutamine residues when these amino acids are part of a peptide structure. L-asparaginase is absent in humans [26].
Many infectious pathogens as well as dysbiosis of the human microbiota are linked to certain autoimmune disorders including RA [29-31]. It has been shown that Escherichia coli secretes L-asparaginase II [32] and that an individual’s microbiome may alter the host L-asparaginase levels [33]. Thus, during infectious processes or dysbiosis, the levels of bacterial L-asparaginase in the human body can be significantly increased. This can lead to posttranslational modifications of many proteins, including CII and the T cell epitopes studied. Therefore, this study aimed to evaluate the ability of bacterial L-asparaginase to modify CII259-273 and CII311-325 T-cell epitopes, and whether the modified epitopes are recognized by ACPAs from RA patients.
We hypothesize that within the “Cit-Gly” motif, ACPAs recognize only the carbonyl moiety (C=O) of the citrulline side chain and not the amino acid citrulline as a whole. We assume that the ACPA-recognizable motif within CII is “carbonyl-Gly-Pro”. Also, this would explain the cross-reactivity of ACPAs with carbamylated and acetylated proteins [13].
Material and methods
Reagents
All reagents, solvents and buffers were purchased from Merck KGaA (Darmstadt, Germany), unless otherwise stated. Ultrapure water (Type I, 18.2 MΩ.cm) was obtained from a PURELAB Ultra Water Purification System (ELGA Lab Water, High Wycombe, UK). Acetonitrile and methanol (Optima LC/MS Grade), and chloroform (HPLC Grade) were purchased from Thermo Fisher Scientific (Fair Lawn, NJ, USA).
Antigens and in vitro enzymatic modifications
Human collagen type II protein (huCII) and L-asparaginase (L-ASNase) from Escherichia coli were purchased from Merck KGaA (Darmstadt, Germany). Synthetic CII peptides (15 amino acids) representing T-cell epitopes within huCII (CII259-273, GIAGFKGEQGPKGEP and CII311-325, APGNRGFPGQDGLAG) were synthesized by Schafer-N (Copenhagen, Denmark). Galactosylated CII259-273 peptide (Gal264-CII259-273) with a β-D-galactopyranosyl residue on L-hydroxylysine at position 264 (GIAGFK[Gal-Hyl]GEQGPKGEP) was synthesized by Syngene (Bangalore, India). A synthetic peptide derived from fibrinogen (alpha chain, Fib29-43) with a citrulline residue at position 35 (AEGGGV[Cit]GPRVVERH, Cit35-Fib29-43, Schafer-N, Copenhagen, Denmark) was used as a positive control. Two irrelevant synthetic peptides (Cldn72–87 TTWYSSVDQLDLRVLQ and Cldn157-174 YNIHLNKKFEPVFSFDYA) representing sequences from the protein claudin-12 that contain target residues for L-asparaginase (glutamine /Q/ and asparagine /N/) were synthesized by Schafer-N (Copenhagen, Denmark). The purity of all synthetic peptides was > 95%.
Human CII and the synthetic peptides (1 mg/ml) were incubated with bacterial L-asparaginase (5 units/mg) in 100 mM Tris-HCl, pH 8.2, for 1 h at 37°C in a total volume of 1 ml. The reactions were stopped by adding 10 mM EDTA. To remove the bacterial L-asparaginase from the reaction mixtures, 10K (for treated peptides) and 50K (for treated huCII) Amicon Ultra-4 Centrifugal Filter Units (Merck KGaA, Darmstadt, Germany) were used. All filters were prewashed with 1 ml of 100 mM Tris-HCl, pH 8.2. Treated samples were transferred to Amicon filters and centrifuged at 4000 ×g for 10 min.
Treated huCII was collected from the 50K centrifugal filter. Protein concentrations of the treated and non-treated huCII were measured on a NanoDrop 2000 UV-Vis spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA) and adjusted to 1 mg/ml in 100 mM Tris-HCl, pH 8.2 for further experiments. For the LC-MS analysis, one part of the collected CII peptide filtrates was diluted 11 times with ultrapure water and 0.3 µl was injected into the chromatographic system.
Peptide analysis using capillary-LC-MS/MS
The liquid chromatography-mass spectrometry (LC-MS) analysis was performed by a Q Exactive LC-MS/MS system (Thermo Fisher Scientific, Waltham, MA, USA). The peptides were loaded on a capillary column (Kinetex 2.6 µm XB-C18 100 Å, 100 mm × 1 mm, Core-shell Silica (Phenomenex Inc., Torrance, CA, USA) equilibrated with liquid phase A using the Accela LC system (Thermo Fisher Scientific, Waltham, MA, USA) equipped with an Accela quaternary high performance liquid chromatography (HPLC) pump and an Accela autosampler. Liquid phases A (98% water, 2% acetonitrile, and 0.1% formic acid), B (2% water, 98% acetonitrile, and 0.1% formic acid), and C (propyl alcohol) were used. Peptides were eluted at the flow rate of 20 µl/min as follows: 100% A (0-5 min), then a linear gradient from 100% A to 5% A + 95% B (5-60 min), linear gradient to 10% B + 90% C (60-75 min), linear gradient to 100% C (75-90 min), linear gradient to 100% B (90-92 min), linear gradient to 100% A (92-115 min).
250°C H-electrospray ionization (H-ESI) vaporizer temperature, spray voltage of +3.0 kV, ion transfer tube temperature of 350°C, sheath gas pressure 11 psi, auxiliary gas flow 1 arbitrary units were adjusted for the interphase. H-ESI position was optimized for the highest sensitivity working mobile phase flow rate. A top 10 Full MS/Data Dependent-MS2 mode of MS detection was performed in positive ion mode with the following settings: 70,000 full width at half maximum (FWHM) resolution in Full MS from 266 to 2500 m/z, 250 ms maximum trap filling time for MS and 120 ms for MS/MS, and 17,500 FWHM resolution for fragment spectra scans with a 1.6 m/z quadrupole isolation window of precursor ions and 18-40 eV stepped collision energy.
Peptide identification was performed by hand using the high resolution MS and MS/MS spectra. No additional software was used except Xcalibur 2.2 (Thermo Fisher Scientific, Hemmel, UK).
Patients with rheumatoid arthritis and healthy subjects
In the present study, the same ERA patients (n = 12) and healthy subjects (n = 11) who participated in a previous study [24] were enrolled again (Table 1). Moreover, we already had frozen sera from these participants. Inclusion criteria for the patients were: 45-60 years old non-smoking women with disease symptoms no more than 12 months since diagnosis (early RA) and fulfilling the American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) 2010 criteria for the classification of RA [18]. All recruited ERA patients were HLA-DRB1*04:01 positive, ACPA positive (> 50 U/ml) and treated only with methotrexate (up to 20 mg weekly) or corticosteroids (≤ 5 mg/day), but not more than three months. These data were collected from the medical files of the patients from a rheumatologist. Patients’ disease activity was assessed using the disease activity score (DAS28) calculated with the level of C-reactive protein (CRP) on the day of blood sampling, quantified by a Latex agglutination slide test (Humatex, Wiesbaden, Germany). Healthy volunteers (controls) were non-smoking women of the same age (45-60 years old), HLA-DRB1*04:01 positive, without diagnosed inflammatory joint diseases. Participants had received no medication in the last 48 hours before blood collection.
Table 1
Demographic, laboratory, and clinical characteristics of the healthy subjects and early rheumatoid arthritis (RA) patients involved in the study
Levels of the anti-CCP3 antibodies in the sera of enrolled participants were determined using the Quanta Lite CCP 3.1 IgG/IgA ELISA kit (INOVA Diagnostics Inc, San Diego, CA, USA). The rheumatoid factor (RF) was measured by using a Roche/Hitachi Cobas C system (Roche Diagnostics International AG, Rotkreuz, Switzerland).
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Local Ethical Committee at Plovdiv University “Paisii Hilendarski”, Bulgaria (5/10.06.2020). The blood sampling procedure was in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. Written informed consent was obtained from all subjects included in the study.
T cell response and cytokine measurement
To evaluate the T cell reactivity, peripheral blood mononuclear cells (PBMCs) were isolated and stimulated with investigated antigens as previously described [24]. After 5 days of stimulation, the concentration of IL-2 and IFN-γ in the supernatants was measured using LEGEND MAX Human IL-2 and Human IFN-γ ELISA kits (BioLegend, San Diego, CA, USA) according to the manufacturer’s instructions. The absorbance was measured at 450 nm (SpectraMax i3x Multi-Mode Microplate Reader, Molecular Devices LLC, San Jose, CA, USA). All samples were analyzed in triplicate.
Humoral response, enzyme-linked immunosorbent assay
To assess the humoral response and detect specific antibodies against the studied antigens, enzyme-linked immunosorbent assay (ELISA) tests were performed. Frozen sera from our previous study [24] were used. Corning 96 Well ELISA plates (Merck KGaA, Darmstadt, Germany) were coated overnight at 4°C with 5 µg/ml of the L-ASNase-treated or non-treated synthetic peptides, or 50 µg/ml treated or native huCII, or 10 µg/ml bacterial L-asparaginase. Unspecific binding was blocked with 5% heat-inactivated fetal bovine serum (FBS) in Dulbecco’s Phosphate Buffered Saline (DPBS) for one hour at room temperature (RT) and washed twice with ELISA buffer (DPBS supplemented with 0.05% Tween-20). Serum samples (diluted 1 : 100 in ELISA buffer) were added to the plates and incubated for 2 h at RT. Plates were washed four times with ELISA buffer and incubated with 100 µl of peroxidase-conjugated goat anti-Human IgA/IgG/IgM (H + L) Secondary Antibody (1 : 1000, Novus Biologicals, LLC, Centennial, CO, USA) for 1 h at RT. After extensive washing five times with ELISA buffer, plates were developed using 2,2'-azino-di-(3-ethylbenzthiazoline sulfonic acid) [ABTS-RO] (Merck KGaA, Darmstadt, Germany) as a substrate, and the optical density (OD) was read at 405 nm using a SpectraMax i3x Multi-Mode Microplate Reader (Molecular Devices LLC, San Jose, CA, USA). The measured OD values were normalized to an ACPA positive RA control pool (n = 12) with absorbance reactivity set at 1.0 arbitrary units (A.U.).
Competitive inhibition ELISA
To determine whether the antibodies in the sera from RA patients that reacted against L-asparaginase modified CII peptides are ACPAs, we performed an additional inhibition ELISA test. Following the ELISA assay for a humoral response described above, before adding the serum samples from RA patients (diluted 1 : 100 in ELISA buffer) to the coated plates with 5 µg/ml enzyme-modified CII peptides, serum samples were incubated either with 10 µg/ml Cit35-Fib29-43 (competitor) or 10 µg/ml CII259-273 (negative control) for 1 hour at 37°C. The next steps were the same as described earlier.
Statistics
Statistical tests were performed with StatView software (SAS Institute, Carry, NC, USA). Statistical differences in serum levels were analyzed by the Mann-Whitney U-test or Kruskal-Wallis test. The cut-off for positivity was calculated based on the receiver operating characteristic (ROC) curve and area under the ROC curve (AUC) with the 95% confidence interval (CI) and > 90% specificity. Results are presented as mean ± SE from individual determinations with at least three replicates. P values less than 0.05 were considered significant (*p < 0.05, **p < 0.01, ***p < 0.001).
Results
Identification of modifications of the T-cell epitopes after in vitro treatment with L-asparaginase
To examine whether bacterial L-asparaginase (L-ASNase) can modify the asparagine and/or glutamine residues in the immunodominant T cell epitopes CII259-273 and CII311-325, as well as the glutamine residue (Q267) in the galactosylated form of CII259-273 (Gal264-CII259-273), the synthetic peptides were treated in vitro with the enzyme. To identify potential modifications of the enzyme-treated CII peptides, LC-MS/MS analyses were performed. Obtained spectra are shown in Figure 2. The non-treated CII peptides were visible at m/z 707.84, m/z 566.31 and m/z 827.90, respectively. Spectra of the peptide mixture, produced after incubation with bacterial L-asparaginase, showed signals shifted to +1 Da corresponding to deamination (m/z 708.34, m/z 567.18 and m/z 828.40, respectively). Our calculations based on the mass area (MA) ratio indicated that 12% of the CII259-273 peptide, 18% of the CII311-325 peptide and 26% of the Gal264-CII259-273 peptide were modified. An example of the approach used for the MS analyses is provided in the Supplementary Figure 1.
Fig. 2
LC-MS mass spectra (FTMS + pESI, positive ion mode) of the L-ASNase-treated CII peptides. A) CII259-273, B) CII311-325, C) Gal264-CII259-273. After the enzymatic treatment, MS analyses showed the presence of peptide mixtures confirming the modification of the peptides. The signals of the modified peptides are indicated by arrows. LC – liquid chromatography, MS – mass spectrometry, FTMS – Fourier transform mass spectrometry, pESI – probe electrospray ionization, L-ASNase – L-asparaginase, CII – collagen type II, Gal – galactose
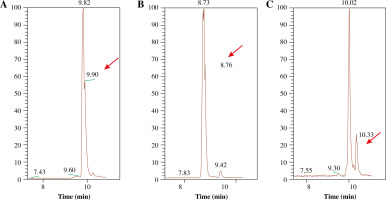
These results demonstrate the role of the bacterial L-asparaginase for posttranslational modifications of huCII, creating novel epitopes that could be responsible for initiating an immune response in RA patients.
T-cell response against non-treated and L-ASNase-treated CII peptides and huCII
To assess whether the bacterial L-asparaginase-treated huCII and collagen peptides, which represent T-cell epitopes, could still induce a T-cell response, leukocytes from the same ERA patients and healthy individuals were stimulated with these antigens for 96 h. As depicted in Figure 3, only Gal264-CII259-273 (regardless of whether it was treated with the enzyme or not) and native huCII were able to induce IL-2 (Fig. 3A) and IFN-γ (Fig. 3B) secretion by the T cells. As expected, the other T cell epitope (CII311-325) cannot activate T cells because it is HLA-DRB1*10 restricted and the blood samples (or antigen-presenting cells) were from patients who express HLA-DRB1*04 molecules. The lack of difference in the T-cell response against non-modified and modified Gal264-CII259-273 leads to the conclusion that the investigated enzyme modification does not affect the amino acids responsible for the binding of the peptide to MHCII or to the T-cell receptor (TCR).
Fig. 3
Production of interleukin 2 (IL-2, A) and interferon γ (IFN-γ, B) by cultured leukocytes from early rheumatoid arthritis (ERA) patients and healthy subjects (control) after in vitro stimulation with non-modified and L-ASNase-treated antigens for 96 h assessed by ELISA. Phytohemagglutinin-L (PHA-L) was used as a positive control. Results are presented as mean ± SE. *p < 0.05, **p < 0.01, ***p < 0.001, as determined by the Mann-Whitney U test
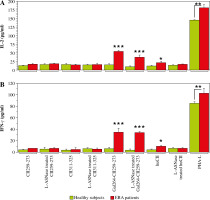
These data indicate that the L-ASNase-modified immunodominant T-cell epitope can still initiate a T-cell response, but now it is also recognized by antibodies present in the sera of ERA patients.
Humoral response to non-modified and L-ASNase-treated CII259-273 peptides
After in vitro treatment of the CII peptides with L-ASNase, we evaluated the humoral response of ERA patients and healthy (control) subjects against non-modified and modified T-cell epitopes. An increased antibody (Ab) titer against L-ASNase-treated CII259-273 peptide was detected in all sera from ERA patients compared to non-modified CII259-273 (Fig. 4A), but it was still lower compared to the Ab levels against Cit35-Fib29-43 (positive control). Most of the control sera were negative for both non-modified and enzyme-treated CII259-273 except subject #2 and subject #6 (Fig. 4B). These two healthy individuals showed a significantly greater Ab response (p = 0.0495) against Cit35-Fib29-43 and L-ASNase-treated CII259-273 compared to the non-modified CII259-273 epitope (Fig. 4B). Summarized results and differences in Ab responses against the studied peptides in ERA patients and healthy controls are presented in Figure 4C. The data showed that ERA patients have antibodies recognizing L-ASNase-treated HLA-DRB1*04-restricted T-cell epitope CII259-273 and citrullinated Fib29-43, but not non-modified CII259-273.
Fig. 4
Reactivity of early rheumatoid arthritis (ERA) sera to non-modified CII259-273 peptide and L-ASNase-treated CII259-273 analyzed by ELISA. A linear Cit35-Fib29-43 peptide (AEGGGV[Cit]GPRVVERH) was used as a positive control. Sera from healthy subjects were used as negative controls. Red lines represent the cut-off thresholds based on the ROC curve (AUC) with 95% confidence interval (CI). Dashed red lines show the maximal positive response of the normalized arbitrary units (A.U.). Results are presented as mean ± SE. *p < 0.05, ***p < 0.001, as determined by the Mann-Whitney U test (two groups) or Kruskal-Wallis test (three groups). Cit – citrulline residue, Fib – fibrinogen, ROC – receiver operating characteristic, AUC – area under the ROC curve
Humoral response to non-modified and L-ASNase-treated CII311-325 peptides
Similarly to the HLA-DRB1*04-restricted T-cell epitope CII259-273, the Ab response against L-ASNase-treated HLA-DRB1*10-restricted T-cell epitope CII311-325 was significantly higher in the sera from ERA patients than the Ab response against non-treated CII311-325 peptide (Fig. 5A).
Fig. 5
Reactivity of early rheumatoid arthritis (ERA) sera to non-modified CII311-325 peptide and L-ASNase-treated CII311-325 analyzed by ELISA. A linear Cit35-Fib29-43 peptide (AEGGGV[Cit]GPRVVERH) was used as a positive control. Sera from healthy subjects were used as negative controls. Red lines represent the cut-off thresholds based on the ROC curve (AUC) with the 95% confidence interval (CI). Dashed red lines show the maximal positive response of the normalized arbitrary units (A.U.). Results are presented as mean ± SE. *p < 0.05, ***p < 0.001, as determined by the Mann-Whitney U test (two groups) or Kruskal-Wallis test (three groups). Cit – citrulline residue, Fib – fibrinogen, ROC – receiver operating characteristic, AUC – area under the ROC curve
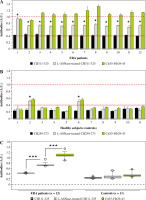
As expected, only sera from the healthy subjects #2 and #6 responded to the enzyme-treated CII311-325 and Cit35-Fib29-43 peptides. No significant difference was observed between the titers of antibodies in the sera from the other healthy (control) subjects (Fig. 5B). The overall humoral response for both groups (ERA patients and healthy controls) to modified and non-modified CII311-325, and to Cit35-Fib29-43, is shown in Figure 5C. Antibody reactivity of ERA patients to two L-ASNase-treated immunodominant T-cell epitopes within CII (CII259-273 and CII311-325) restricted by different MHC II molecules had similar profiles.
Humoral response to non-treated and L-ASNase-treated galactosylated CII259-273 peptides
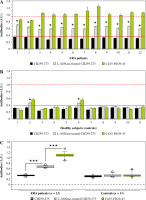
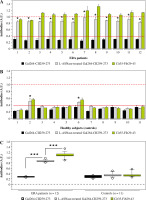
The most common post-translational modification of the major immunodominant T-cell epitope CII259-273 is hydroxylation and subsequent glycosylation at position K264. The T-cell response in RA patients and in mouse models of rheumatoid arthritis (collagen-induced arthritis, CIA) is directed and predominates against this modified form of the recognizable epitope [34-37]. This was the reason to include this peptide in the present study. Treatment of this peptide with bacterial L-asparaginase and its potential modification would result either in its eventual recognition by antibodies (formation of a new B-cell epitope) or loss of its T-cell reactivity (inability to bind to antigen-presenting cells and to activate the autoreactive T cells). The results for the B-cell response observed by ELISA tests are shown in Figure 6. As can be seen from the figure, similarly to the other two peptides, sera from ERA patients showed significant reactivity to the enzyme-modified epitope compared to the non-treated peptide, with values significantly higher than those for the other two peptides. Here again, the sera of two healthy individuals who showed reactivity to the other enzyme-treated collagen peptides showed an elevated antibody titer (Fig. 6B). The B-cell response to L-ASNase-treated CII peptides was most clearly expressed to this peptide, where we measured the highest values (Fig. 6).
Fig. 6
Reactivity of early rheumatoid arthritis (ERA) sera to Gal264-CII259-273 peptide and L-ASNase-treated Gal264-CII259-273 analyzed by ELISA. A linear Cit35-Fib29-43 peptide (AEGGGV[Cit]GPRVVERH) was used as a positive control. Sera from healthy subjects were used as negative controls. Red lines represent the cut-off thresholds based on the ROC curve (AUC) with the 95% confidence interval (CI). Dashed red lines show the maximal positive response of the normalized arbitrary units (A.U.). Results are presented as mean ± SE. *p < 0.05, ***p < 0.001, as determined by the Mann-Whitney U test (two groups) or Kruskal-Wallis test (three groups). Cit – citrulline residue, Fib – fibrinogen, ROC – receiver operating characteristic, AUC – area under the ROC curve
Cit35-Fib29-43 peptide blocks the humoral response to L-ASNase-treated CII peptides
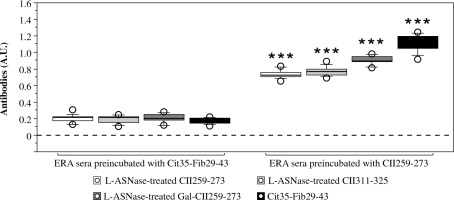
To assess whether the observed humoral response of RA serum samples to the enzymatically modified CII peptides is due to ACPAs or to other antibodies, we performed a competitive ELISA with a citrullinated peptide. No response was detected when the serum samples from ERA patients were preincubated with Cit35-Fib29-43 peptide (Fig. 7). In contrast, sera preincubated with an irrelevant peptide (CII259-273), which does not contain citrulline, reacted to L-ASNase-modified peptides and to the citrullinated peptide (Fig. 7). These results indicate that ACPAs cross-react with the modifications caused by the bacterial L-asparaginase.
Fig. 7
Reactivity of early rheumatoid arthritis (ERA) sera preincubated with citrullinated (Cit35-Fib29-43) or noncitrullinated (CII259-273) peptide to L-ASNase-treated CII peptides analyzed by ELISA. Results are presented as mean ± SE. ***p < 0.001, as determined by the Mann-Whitney U test (two groups) for each peptide (sera preincubated with CII259-273 vs sera preincubated with Cit35-Fib29-43). Cit – citrulline residue, Fib – fibrinogen
Humoral response to non-treated and L-ASNase-treated human type II collagen (huCII)
Besides the studied T-cell epitopes, the human CII protein (huCII) contains a number of B-cell epitopes, with proven reactivity of sera derived from patients with rheumatoid arthritis [38-43]. Treatment with bacterial L-asparaginase can lead to creation of neoepitopes different from the known T- and B-cell epitopes. The reactivity of sera from ERA patients and healthy subjects against L-ASNase-treated and untreated huCII is presented in Figure 8. Data showed that sera from ERA patients reacted to both native huCII and enzyme-modified huCII. Antibody reactivity to L-ASNase-treated huCII was significantly higher compared to the non-treated huCII (p = 0.0002).
Fig. 8
Reactivity of early rheumatoid arthritis (ERA) sera to native human collagen type II protein (huCII) and L-ASNase-treated huCII analyzed by ELISA. Bacterial L-asparaginase was used as a negative control. Sera from healthy subjects were used as negative controls. Results are presented as mean ± SE. ***p < 0.001, as determined by Kruskal- Wallis test
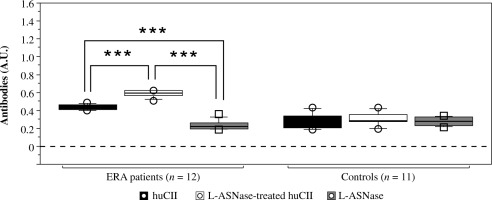
The detected lower humoral response to native huCII was still significant and above the background compared to the Ab response against L-asparaginase (Fig. 8). None of the control sera reacted to the tested proteins (huCII or L-asparaginase).
These results indicate that sera from ERA patients recognize several epitopes on huCII and the number of these epitopes increases after treatment with bacterial L-asparaginase.
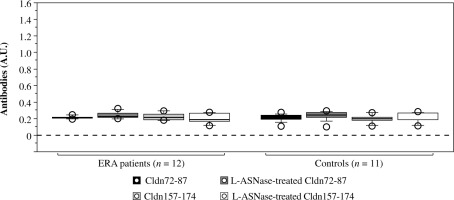
Humoral response to non-treated and L-ASNase-treated irrelevant synthetic claudin peptides
In order to investigate whether bacterial L-ASNase can create other B-cell epitopes recognized by antibodies from anti-CCP positive ERA patients, we treated in vitro with the enzyme two irrelevant peptides from the claudin-12 protein (Cldn72–87 TTWYSSVDQLDLRVLQ and Cldn157-174 YNIHLNKKFEPVFSFDYA) that contain glutamine or asparagine residues (targets for the L-ASNase). As can be seen in Figure 9, no anti- body reactivity was detected in either ERA patients or healthy controls. An explanation for these results could be that the main motif (carbonyl-Gly-Pro) recognized by ACPAs here is missing. Therefore, not all posttranslational modifications by bacterial L-ASNase lead to the creation of epitopes which are targets for ACPAs. Such antibody reactivity requires the presence of a “carbonyl-Gly-Pro” motif. This sequence occurs repeatedly in type II collagen.
Fig. 9
Reactivity of early rheumatoid arthritis (ERA) sera to non-treated and L-ASNase-treated irrelevant synthetic peptides (Cldn72–87 TTWYSSVDQLDLRVLQ and Cldn157-174 YNIHLNKKFEPVFSFDYA) analyzed by ELISA. Sera from healthy subjects were used as negative controls. Results are presented as mean ± SE. Cldn – claudin-12 protein
Discussion
We have already reported that bacterial L-asparaginase can activate the T-cell response in ERA patients through molecular mimicry [24]. As an extension of that study, we report here that this enzyme may also play a role in the B-cell response by modifying immunodominant T-cell epitopes.
The bacterial L-asparaginase has both glutaminolytic and asparaginolytic activities and belongs to the glutaminase-asparaginase family (EC 3.5.1.38). Compared to the asparaginolytic activity, the efficiency of L-glutamine hydrolysis is about 0.01% [25]. Our LC-MS/MS analyses revealed that only 12% of the CII259-273 peptide (containing one glutamine residue), 18% of the CII311-325 peptide (containing one glutamine and one asparagine residues) and 26% of the Gal264-CII259-273 peptide (containing one glutamine residue) were modified by the enzyme. Yet, this is sufficient to alter the studied epitopes and make them recognizable by antibodies in sera from ERA patients. These antibodies are anti-citrullinated protein antibodies (ACPAs) cross-reacting with the “carbonyl- Gly-Pro” motif of the modified T-cell epitopes. First, all sera that reacted to L-ASNase-treated CII peptides were anti-CCP3 positive [24]. Second, all of them recognized the citrullinated fibrinogen peptide (Cit35-Fib29-43) that was used as a positive control, and the antibody reactivity to this peptide was significantly higher compared to the other CII peptides. Third, the common structure that results after posttranslational modifications of the investigated peptides is the carbonyl group of glutamate, aspartate or citrulline, followed by the amino acids glycine (Gly, G) and proline (Pro, P). Fourth, the humoral response towards treated huCII was significantly higher compared to the native huCII (Fig. 8). Fifth, no antibody reactivity was detected against L-ASNase-modified irrelevant peptides that did not contain a “carbonyl-Gly-Pro” motif. Finally, our competitive inhibition ELISA test showed that the Cit35-Fib29-43 peptide blocked antibody reactivity to L-ASNase-treated CII peptides. All these data confirm our hypothesis that ACPAs recognize the “carbonyl-Gly-Pro” motif within CII.
Several studies have demonstrated that ACPAs from RA patients recognize multiple citrullinated antigens [20, 23, 42, 44-46] and this recognition depends more on the structural homology of the epitopes than on their sequence homology [42, 47]. Due to the wide range of citrullinated proteins identified in various samples from RA patients, the term ‘citrullinome’ was recently introduced [10, 46, 48]. On the other hand, it was stated that citrullination is a normal physiological process that modulates the activity of many metabolic enzymes, which regulate various cellular functions [48]. Therefore, citrullination is not specific to rheumatoid arthritis or other autoimmune disease; nor does it necessarily cause ACPA formation. Thus, the question about the mechanism of development of ACPAs and their role in the pathogenesis of RA remains open.
In our study, we detected increased levels of antibodies against L-ASNase-treated CII peptides and Cit35-Fib29-43 peptide (Figs. 4-6) in two healthy subjects (#2 and #6). Although the antibody levels were lower compared to those in ERA patients, these data demonstrate the prognostic value of the ACPAs concerning RA onset in asymptomatic subjects [17]. Since these healthy volunteers did not report the presence of other diseases, it will be interesting to follow them over the next few years to see if they will develop RA.
Besides ACPAs that cross-react with various citrullinated proteins, in recent years special attention has been paid to anti-carbamylated protein antibodies (anti-CarPAs) and anti-acetylated protein antibodies (AAPAs), which also cross-react with various citrullinated antigens [10, 13, 16, 21, 49]. To distinguish antibodies with overlapping reactivity (against citrullinated, carbamylated and acetylated proteins) the term anti-modified protein antibodies (AMPAs) was introduced [21, 49]. In our study, it is likely that such AMPAs present in the sera from RA patients reacted towards L-ASNase-modified CII T-cell epitopes, but not towards the L-ASNase-modified irrelevant claudin peptides. Investigated T-cell epitopes do not contain a citrulline residue, but after treatment with bacterial L-ASNase are recognized by these AMPAs. On the other hand, sera were from anti-CCP3 positive ERA patients and also reacted against the citrullinated peptide (Cit35-Fib29-43). In addition, research has shown that the central Cit-Gly motif in combination with the peptide backbone is essential for ACPA reactivity [47]. Taken together, these data indicate that AMPAs cross-react with the “carbonyl-Gly-Pro” motif of the L-ASNase modified CII peptides, where the carbonyl group (C=O) of glutamate/aspartate residues (and most likely from citrulline) is crucial for the antibody recognition. This motif is common for the L-ASNase-treated CII peptides (CII259-273 [GIAGFKGEEGPKGEP], CII311-325 [APGDRGFPGEDGLAG], Gal264-CII259-273 [GIAGFK[Gal-Hyl]GEEGPKGEP]) and for the citrullinated peptide (Cit35-Fib29-43 [AEGGGV[Cit]GPRVVERH]) used as a positive control. This motif was absent in the L-ASNase-modified irrelevant synthetic claudin peptides (Cldn72–87 TTWYSSVDELDLRVLE and Cldn157-174 YDIHLDKKFEPVFSFDYA), where no AMPA recognition was detected.
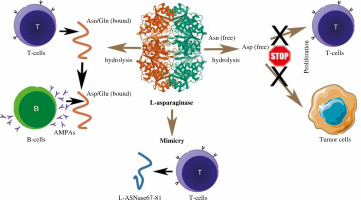
So far, periodontitis and gut dysbiosis have been suggested to be related to protein PTMs (citrullination, acetylation) and RA pathogenesis, respectively [21, 50-52]. This study demonstrated that bacterial L-asparaginase, which could be produced by different bacterial strains, also contributes to the PTMs of proteins, and all these modifications are recognized by AMPAs. In the concept of the “first hit” (appearance of ACPAs), the missing piece is how T cells participate at the early stage. This concept presumes that during the “second hit”, HLA “shared epitope” (HLA-SE) associated T-cells could recognize citrullinated antigens presented by the B cells, providing them further help for expansion and evolution [21]. For as much as AMPAs cross-react with different PTMs, it is not necessary for T-cells to recognize citrullinated epitopes. Our results showed that bacterial L-asparaginase can modify the known immunodominant T cell epitopes, making them recognizable by ACPAs/AMPAs, and at the same time they are still recognized by T cells as well (Fig. 3), because the modifications do not alter amino acids that are MHCII or TCR contact points [24]. Our hypothesis for the initiation of RA is that 1) multiple bacterial infections could prime the T cell response through molecular mimicry; 2) at the same time these pathogens cause enzymatic PTMs of the target proteins; 3) by receiving help from the activated T cells, B cells begin to produce AMPAs that cross-react with different posttranslational modifications initiating destruction of the self-tissues containing these modifications. Summarized functions of the bacterial L-asparaginase are presented in Figure 10.
Fig. 10
Summary of the L-asparaginase activities and mechanisms of action. Asn – Asparagine, Asp – aspartic acid, Gln – glutamine, Glu – glutamic acid, AMPAs – anti-modified protein antibodies
Bacterial enzymes (including L-asparaginase and peptidyl arginine deiminase – PAD) are fully capable of mediating RA-specific posttranslational modifications of target proteins such as CII. In combination with molecular mimicry of key immunodominant epitopes, many bacterial antigens could induce an autoimmune response. Furthermore, the fact that bacterial L-asparaginase has recently been classified as a moonlighting protein (http://www.moonlightingproteins.org/protein_detail/?mpid=455, accessed on 17 September 2022) should not be overlooked. Many of these additional activities of the moonlighting proteins are related to the virulence and adhesion properties of the bacterial pathogens towards the host extracellular matrix, modulation of leukocyte activity and evasion of the host immune response [53, 54]. Therefore, such proteins are potential initiators of many autoimmune diseases.
Our study has certain limitations due to the small sample size, which included only non-smoking, HLA-DRB1*04:01 positive, female RA patients and healthy subjects. Despite the contribution of our findings about the possible role of bacterial L-asparaginase and ACPAs/AMPAs in the initiation and pathogenesis of RA, these data cannot be extrapolated to all RA patients, especially to the HLA-DRB1*04:01 negative patients, smokers or male RA patients. Furthermore, the development of most autoimmune diseases begins 3-5 years before the corresponding diagnosis. Therefore, the presence of infection and correspondingly increased levels and activity of bacterial L-asparaginase is not necessary for development of the disease after the diagnosis in RA patients. The immune system could be primed years before the diagnosis, and this is valid for the ACPAs/AMPAs as well. They appear several years before the development of RA. Thus, most changes of the self-antigens/epitopes (if there are such), which are responsible for the initiation of the disease, have already occurred. Further extension of research on this topic may contribute to the understanding of the role of bacterial L-asparaginase during bacterial infections in the initiation of RA. Measuring ACPA/AMPA levels after bacterial infections can be included in the clinical practice as a predictive biomarker for early diagnosis of RA.
Conclusions
The current study demonstrated that bacterial L-asparaginase can modify the major RA-associated immunodominant T cell epitopes within CII, hydrolyzing glutamine/asparagine residues to glutamic and aspartic acids, respectively. The modified epitopes are recognized by cross-reactive ACPAs/AMPAs present in the sera of anti-CCP3 positive ERA patients bearing HLA-DRB1*04:01 alleles. We hypothesize that ACPAs/AMPAs recognize the “carbonyl-Gly-Pro” motif within CII. These PTMs do not affect the T-cell recognition of the studied epitopes. Our findings extend the knowledge and understanding about the role of enzymatic PTMs of proteins in the initiation and pathogenesis of RA.


