Introduction
According to a worldwide cardiac pacing and implantable cardioverter-defibrillators (ICDs) survey, 1.25 million pacemakers and 410 000 ICDs were implanted worldwide in the year 2009 [1]. More than 547 000 pacemakers and 105 000 ICDs in the 52 European Society of Cardiology (ESC) member countries and more than 87 000 cardiac resynchronization therapy (CRT) devices in the 46 ESC member countries were implanted in the year 2016 [2]. Data from clinical studies or surveys indicate that the rate of long-term anticoagulation use is 15–35% in patients with a pacemaker or implantable cardioverter-defibrillator, reaching up to 50% in patients with CRT devices [3–5]. Peri-procedural management of anticoagulation therapy is complicated as it poses a challenge of balancing between thromboembolism (TE) and bleeding risk. Prolonged interruption of anticoagulants might result in a time period of subtherapeutic anticoagulation. However, uninterrupted anticoagulation could increase the risk of bleeding, and device pocket haematoma is one of the most common complications associated with cardiac rhythm management device surgery with a reported incidence of 2.1–9.5% [6]. Device pocket haematoma is defined as ecchymosis or bleeding in the area of the device generator pocket [7]. The formation of a haematoma is of clinical importance due to its association with an increased device pocket infection risk [8, 9]. Moreover, in the case of evidence of clinically significant device pocket haematoma formation, extended interruption of anticoagulation may be necessary, which could also result in increased thromboembolic risk. It is also evident that device-related haematoma is associated with increased length of hospital stay and higher in-hospital mortality of 2.0% [10]. Therefore, it is important to optimize the anticoagulation therapy during the peri-procedural device implantation period.
For many decades, vitamin K antagonists (VKAs) have been the mainstay of anticoagulation for various conditions, including atrial fibrillation (AF). In order to avoid thromboembolic complications during cardiac device surgery, bridging with unfractionated heparin (UH) or low molecular weight heparin (LMWH) used to be the standard of care for patients with a moderate to high TE risk. Eventually it was noticed that such a strategy implies a significantly higher clinically significant device pocket haematoma risk [11]. Non-vitamin K antagonist oral anticoagulants (NOACs) proved to be non-inferior to VKAs in preventing ischaemic events in patients with atrial fibrillation with a favourable risk-benefit profile driven by a reduction in haemorrhagic stroke risk [12]. Therefore, NOACs are the preferred first-line treatment for preventing systemic embolization and stroke in patients with AF [13]. In spite of non-vitamin K antagonist oral anticoagulants gaining popularity, the strategies of peri-procedural management of NOACs during cardiac implantable device surgery vary because unequivocal recommendations are lacking. In addition, patients requiring cardiac device surgery have more comorbidities and require additional medications. More precisely, up to 50% of patients elected for cardiovascular implantable electronic devices (CIEDs) procedures require antiplatelet therapy [3–5].
Principally, the selection of anticoagulant strategy is guided by general recommendations based on the risk of bleeding caused by the invasive procedure, but such classification does not fully take into account patient-specific factors and the circumstances of a certain procedure [14]. An increasing number of studies describing the management of anticoagulants during specific interventional procedures provide more evidence-based data for the management of these drugs during CIED procedures.
Aim
Thus, the aim of this review is to describe the strategies for the use of different anticoagulants and to systematically assess their safety and efficacy in patients undergoing CIED surgery.
Material and methods
A systematic literature search was conducted in PubMed, Web of Science and Cochrane Central Register of Controlled Trials (CENTRAL) bibliographic databases (from inception to April 2023) with no language limitations. The search was performed from January 2022 to April 2023. The search string used was: “anticoagulant therapy” OR “anticoagulation” OR “NOACs” OR “rivaroxaban” OR “apixaban” OR “edoxaban” OR “dabigatran” OR “vitamin K antagonists” OR “VKAs” AND (“cardiovascular implantable electronic devices” OR “CIEDs” OR “pacemaker” OR “ICDs”).
Randomized controlled trials or observational studies published in English language were included. Selected studies included patients on long-term anticoagulation therapy who underwent cardiovascular implantable electronic device surgery. Publications were selected based on predefined inclusion and exclusion criteria (Table I) and reviewed by the two authors (AD, GR). Agreement of the two reviewers was required for studies to be included. There were no disagreements between the authors.
Table I
Articles eligibility criteria
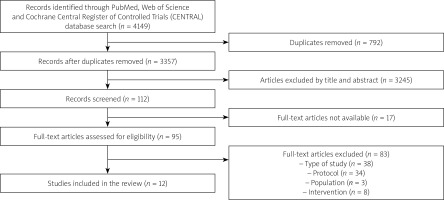
A total of 4149 bibliographic entries were found. Bibliographic references were managed with the Zotero program. Compliance of the articles with the selection criteria was assessed in two stages. During the first stage, duplicates were removed and studies were selected that, according to the title and abstract, possibly met the study selection criteria. A total of 112 publications were selected during this stage. During the second stage, the compliance of the studies with the established criteria was assessed by analysing full-text articles. A total of 12 articles were selected, the results of which were described and presented in tables. The article selection process and its stages are presented in Figure 1.
Figure 1
Flow chart of article selection process. Adapted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) framework [55]
Information on 1) study design, 2) sample size, 3) concomitant antiplatelet therapy, 4) intervention strategies (including interruption or continuation of oral anticoagulants, bridging therapy, anticoagulation interruption periods, follow-up duration), 5) outcome measures (bleeding and thromboembolic events) were extracted and presented.
Results
Description and results of the studies
Taking into account the strategy of using anticoagulants during the implantation of cardiac rhythm regulating devices and the comparative groups evaluated in the studies, 4 groups of studies were distinguished. Due to the heterogeneity of research methodologies, descriptions of each group’s methods and results are presented below.
The strategy of continuing vitamin K antagonists during cardiac electronic device implantation is compared with the strategy of interruption
Two studies were assigned to this group. In these studies, VKAs were used in all of the study population. In a randomized clinical trial by Birnie et al., patients were randomly assigned to continued warfarin treatment or bridging therapy with heparin. For patients receiving bridging therapy, warfarin was discontinued 5 days before the procedure and they started receiving therapeutic doses of LMWH or intravenous heparin 3 days before the procedure. The final dose of LMWH was administered > 24 h before the procedure. Heparin infusion was discontinued at least 4 h before surgery [11]. In a randomized clinical trial by Airaksinen et al., patients were assigned to continued warfarin treatment or warfarin interruption 2 days before the procedure without bridging [15].
Safety and efficacy of continuation or interruption of vitamin K antagonist oral anticoagulants
In a study by Birnie et al., the primary outcome was clinically significant device-pocket haematoma, defined as a haematoma requiring further surgery, resulting in prolongation of hospitalization, or requiring interruption of oral anticoagulation therapy. Clinically significant haematoma occurred in 12 of 343 patients (3.5%) in the continued-warfarin group as compared with 54 of 338 (16.0%) in the heparin-bridging group (relative risk = 0.19; 95% confidence interval: 0.10 to 0.36; p < 0.001). In a study by Airaksinen et al., the primary study outcome measure included major bleeding events and thromboembolic complications. Major bleeding was defined as any bleeding or pocket haematoma that required additional intervention or discontinuation of anticoagulation. Major bleeding events were rare in both groups. Only 1 patient in the uninterrupted warfarin group needed blood transfusion (2 units of red blood cells due to rupture of proximal cephalic vein).
The strategy of continuing non-vitamin K antagonist oral anticoagulants during cardiac device implantation is compared with the strategy of interruption
Three studies were assigned to this group. In all of these studies, NOACs were used in all of the study population. In randomized clinical trials by Birnie et al. and Ricciardi et al., dabigatran, rivaroxaban and apixaban were used [16, 17]; in a prospective cohort study by Unverdorben et al., edoxaban was used [18]. In one comparative group, NOACs were continued during the perioperative period of device implantation, with the last dose administered either the evening before the procedure or the morning of the procedure. Anticoagulants were continued after the procedure without missing a single dose. In the second comparative group, NOACs were discontinued for 24 h or longer before the procedure, depending on the specific anticoagulant, patients’ glomerular filtration rate (GFR) and the methodology used in the study [19].
Safety and efficacy of continuation or interruption of non-vitamin K antagonist oral anticoagulants
The primary outcome assessed by Birnie et al. and Ricciardi et al. was clinically significant device pocket haematoma. It is defined as a haematoma requiring revision, temporary discontinuation of anticoagulants or use of an antidote (resulting in at least 24 h of sub-therapeutic anticoagulation) or prolonged hospitalization (at least 24 h after the procedure or requiring re-hospitalization for haematoma) [11]. In a prospective cohort study by Unverdorben et al., the primary outcome was major bleeding according to the International Society on Thrombosis and Haemostasis (ISTH) definition [18]. In a study by Birnie et al., clinically significant haematoma developed in 7 out of 328 patients (2.1%; 95% CI: 0.9–4.3) in the continued NOACs group and in 7 out of 334 (2.1%; 95% CI 0.9–4.3) in the interrupted NOACs group (p = 0.97). According to Ricciardi et al., clinically significant haematoma developed in only 1 patient on continued NOACs (2%) and there were no primary outcomes in the interrupted NOACs group (p = 0.320). Unverdorben et al. observed no primary outcomes in either group. 1 (0.3%) stroke was observed in both groups in a study by Birnie et al. and no thromboembolic events occurred in other studies. There were no statistically significant differences in primary and secondary outcomes between the evaluated groups.
The strategy of NOACs cessation compared to the use of VKAs during cardiovascular implantable electronic device procedures
Three studies were assigned to this group. In all these studies, one comparative arm consisted of patients in whom NOACs were interrupted in the peri-procedural period. Studies by Essebag et al. and Madan et al. evaluated discontinuation of dabigatran [20, 21]. In a post-hoc analysis by Essebag et al., according to the clinical trial protocol, it was recommended to stop dabigatran for at least 24 h with normal renal function, at least 48 h if creatinine clearance (CrCl) is 30–50 ml/min, 2–4 days when CrCl is < 30 ml/min and resume when adequate haemostasis is achieved. Dabigatran was discontinued < 24 h in 37 (9.0%) patients, and < 12 h in 1 (0.2%) patient due to non-adherence to the study protocol. Also, 56 (13.7%) patients received pre-procedural bridging therapy after stopping oral anticoagulant therapy, and 41 (10.0%) received post-procedural bridging. In a study by Madan et al., dabigatran use was considered interrupted when the last dose was taken ≥ 12 h before the procedure. In a study by De Heide et al., dabigatran, edoxaban, rivaroxaban, and apixaban were evaluated in the NOACs arm, with the last dose administered 24–48 h before the procedure depending on renal function [21]. Another comparative arm consisted of patients taking VKAs. In studies by De Heide et al. and Madan et al., VKAs were continued, maintaining the international normalized ratio (INR) in the therapeutic window. In a study by Essebag et al., 37 (18.4%) patients in the VKAs arm received pre-procedural bridging and 37 (18.4%) patients received post-procedural bridging.
Safety and efficacy of NOACs interruption compared with VKAs
The primary outcome in a study by De Heide et al. was clinically significant device pocket haematoma (defined previously). No statistically significant difference was observed between the comparison arms (p = 0.33). The primary endpoint evaluated in a study by Madan et al. was major bleeding, defined as haemothorax, haemopericardium, intracranial haemorrhage, gastrointestinal bleeding, epistaxis, or device pocket haematoma requiring surgical intervention. There was no statistically significant difference between the groups (p > 0.99). In a study by Essebag et al., evaluated outcomes were device pocket haematoma, major bleeding according to ISTH definition, and thromboembolic complications – ischaemic stroke, SE, myocardial infarction, pulmonary artery thromboembolism and death. Device pocket haematoma was defined as all bleeding complications involving the cardiovascular implantable electronic device surgical wound. There were no statistically significant differences between complications observed in the dabigatran and warfarin groups. However, in a sub-analysis based on the use of bridging in the warfarin arm, the rate of device pocket haematoma was 10.8% with bridging versus 2.4% without bridging. The incidence of device pocket haematoma was lower in the dabigatran group compared to warfarin with bridging (risk difference: –8.62%, 95% CI: –24.15 to –0.51; p = 0.034), but not compared to warfarin without bridging (risk difference = –0.24%, 95% CI: –4.20 to 2.33; p = 0.880). Only Essebag et al. observed thromboembolic events, but there was no statistically significant difference between the groups.
The strategy of NOACs continuation or interruption is compared with the use of VKAs
Four studies were assigned to this group. In these studies, one comparative arm consisted of NOACs that were either continued or interrupted peri-procedurally, and some patients received heparin or LMWH bridging therapy. Another group consisted of patients receiving VKAs. Leef et al. evaluated rivaroxaban, which was temporarily discontinued in the majority of patients. Warfarin was also discontinued in most cases, with bridging therapy in only a small number of patients [22]. In a study by Black-Maier et al., comparison groups were similar except that all NOACs were assessed, and timing of anticoagulant discontinuation was not reported [23]. Steffel et al. divided the population into high-dose edoxaban regimen (HDER, 60 mg edoxaban per day), low-dose edoxaban regimen (LDER, 30 mg edoxaban per day), and warfarin groups. Anticoagulation was considered interrupted when doses were missed for more than 3 consecutive days. No bridging therapy was applied in any group [24]. Pillarisetti et al. assigned patients receiving dabigatran, rivaroxaban, or apixaban to the NOACs group. Only one dose of apixaban and dabigatran was omitted before the procedure, while rivaroxaban was administered continuously. Warfarin was not discontinued for more than 48 h, and bridging was not applied [25].
Safety and efficacy of NOACs continuation or interruption strategies compared with VKAs
In a study by Leef et al., primary efficacy outcomes assessed were stroke or systemic embolization, and primary safety outcomes were major or non-major clinically significant bleeding according to ISTH. The number of events in the rivaroxaban and warfarin groups was too small for formal hypothesis testing, and the primary outcome rates were similarly low when evaluating the continued and interrupted anticoagulant groups. Black-Maier et al. assessed the rates of major bleeding, stroke or transient ischaemic attack (TIA), hospitalization for bleeding, cardiovascular causes, or all-cause hospitalization. Complications were rare regardless of anticoagulation strategy. Primary outcomes assessed in a study by Steffel et al. were stroke or systemic embolism, and major bleeding according to ISTH. The rates of ischaemic and bleeding events were infrequent and not statistically significantly different between the comparison groups. In a study by Pillarisetti et al., the primary endpoint was major haematoma within 1 week of the procedure. It was defined as any haematoma that resulted in re-exploration or drop in the haemoglobin level of > 2 g/dl or required blood transfusion or increased the length of hospital stay or resulted in interruption of anticoagulation therapy. There was also no statistically significant difference of the primary and secondary endpoints between groups. The baseline characteristics and anticoagulation interruption periods are presented in Table II. The results of all studies are presented in Table III.
Table II
Baseline characteristics of included studies
[i] DAPT – dual antiplatelet therapy, GFR – glomerular filtration rate, HDER – high dose edoxaban regimen (60 mg edoxaban per day), ISTH – International Society on Thrombosis and Haemostasis, LDER – low dose edoxaban regimen (30 mg edoxaban per day), NOACs – non vitamin-K antagonist oral anticoagulants, N/A – not analysed, VKAs – vitamin K antagonists.
Table III
Results of included studies
[i] CI – confidence interval, HDER – high dose edoxaban regimen (60 mg edoxaban per day), ISTH – International Society on Thrombosis and Haemostasis, LDER – low dose edoxaban regimen (30 mg edoxaban per day), NOACs – non vitamin-K antagonist oral anticoagulants, N/A – not analysed, SE – systemic embolism, VKAs – vitamin K antagonists.
Discussion
Interpretation of study results
Most of the reviewed studies found no statistically significant difference between the safety and efficacy outcomes of anticoagulation strategies. However, most of these studies were not sufficiently powered to detect these differences due to the small number of events. Such results are consistent with the results of previous studies. Jennings et al. aimed to determine the risk of bleeding and thromboembolic complications associated with continuous anticoagulation (comparing 48 patients on dabigatran with 195 patients on warfarin) during cardiac device implantation. The incidence of bleeding complications was not statistically significantly different between the groups (2.1% in the dabigatran group, 4.6% in the warfarin group, p = 0.69) [26]. In a prospective observational study of 25 patients receiving dabigatran who underwent cardiac electronic device implantation by Rowley et al., no major bleeding events or thromboembolic complications were observed [27]. An increased risk of bleeding was found in studies by Birnie et al. and Essebag et al. in the warfarin group in those patients who received bridging therapy. This finding also corresponds to the results of previously performed meta-analyses [28, 29]. Regarding efficacy outcomes of anticoagulation strategies, the peri-procedural risk of stroke/systemic embolization reported in the studies discussed in this review was 0–1.26%.
The use of vitamin K antagonists during implantable cardiac rhythm management device procedures
The results of the randomized clinical trial Bridge or Continue Coumadin for Device Surgery Randomized Controlled Trial (BRUISE CONTROL) showed that clinically significant device pocket haematoma developed 4.6 times less frequently without discontinuing warfarin compared to warfarin discontinuation and bridging therapy, even though this was the standard of care prior to this study [11]. This study confirmed the results of previous observational studies and meta-analyses [29–31]. Therefore, according to the consensus of ESC experts, it is now recommended to perform cardiac device implantation without interrupting VKAs, and if the annual risk of thromboembolism is < 5%, to stop their use 3–4 days before the procedure without using bridging or to continue VKAs [32].
Most studies evaluating perioperative anticoagulation strategies include patients of moderate to high thromboembolic risk, and when low-risk patients are included, they are usually assigned to a control group without using bridging. Although the annual thromboembolic risk in such patients is low, this risk increases in the perioperative period, and discontinuation and reinitiation of anticoagulants may also create a transient prothrombotic state [33]. Hence, the ESC pacing and cardiac resynchronization therapy guidelines recommend that VKAs be continued periprocedurally without the use of bridging [6]. However, specifically designed randomized clinical trials are required to provide reasonable evidence as to whether VKAs should be continued or interrupted in the periprocedural period in patients at low thromboembolic risk.
In regard to patients with mechanical heart valves (MHVs), it is currently recommended to perform device surgery without interruption of VKAs. This applies to patients with a prosthetic mitral valve, cage ball or tilting disc aortic valve and bileaflet aortic valve prothesis and AF and a CHA2DS2-VASc score of ≥ 2 and also to patients with recent venous thromboembolism (within 3 months) and severe thrombophilia. The INR should be monitored and maintained at or below the upper limit of the therapeutic range (usually ≤ 3 or ≤ 3.5 for some prosthetic heart valve patients) [32]. Data regarding the patients at very high thromboembolic risk (patients with a mechanical mitral valve prosthesis or multiple mechanical valve prostheses, atrial fibrillation and/or a history of stroke/TIA) are not yet conclusive. In all situations, the risks of bleeding should be weighed against the benefits of thromboembolism prevention. Of the reviewed studies, only Black-Maier et al. described complications in patients with valvular AF. Patients who suffered stroke/TIA in the peri-implant setting had an average CHA2DS2-VASc of 5, and 2 of 3 (66%) had valvular AF (1 was managed with uninterrupted warfarin, 1 with interruption and bridging, and 1 interrupted without bridging).
Despite data from previous studies showing a higher rate of bleeding complications, bridging with heparin or LMWH is still common in patients with atrial fibrillation when warfarin is stopped before the procedure. In some of the analysed studies, the use of parenteral anticoagulants was evaluated as an exclusion criterion, while in others this strategy was applied to a significant percentage of patients. In the studies included in the review, this method was used in 14.9% to 18.4% of patients in whom warfarin was interrupted [20, 22, 23]. In the prospective multicentre survey European Snapshot Survey on Procedural Routines for Electronic Device Implantation (ESS-PREDI, 2016), bridging with heparin was used in 55 out of 154 (35.8%) patients. Since less than 10% included patients had a valve prosthesis/heart valve disease or a prior stroke/TIA, the authors of the survey do not believe that this could have influenced the results, and consider such a percentage as non-compliance with the guidelines [34]. However, some authors note that in the studies which found increased risk of bleeding associated with bridging, the population at a very high risk of thromboembolism was not properly reflected. In such situations, the authors call for a careful assessment of the benefit-risk ratio when choosing a treatment strategy [35].
Non-vitamin K antagonist oral anticoagulants use during implantable cardiovascular implantable electronic device procedures
Non-vitamin K antagonist oral anticoagulants include the factor Xa inhibitors apixaban, edoxaban, and rivaroxaban and the direct thrombin inhibitor dabigatran. Knowing that the prevalence of atrial fibrillation is high among patients for whom implantation of a cardiac device is indicated, and that the use of NOACs is increasing, more evidence is needed for the application of these drugs in specific clinical situations. For procedures with the lowest risk of bleeding (minor risk interventions), including the cardiovascular implantable electronic device procedures, the practical guide of the European Heart Rhythm Association (EHRA, 2021) recommends that the last dose of NOACs be taken 12–24 h before the scheduled procedure, and their use be continued 6 h after the procedure (skipping one dose of dabigatran and apixaban and continuing edoxaban or rivaroxaban) [14].
The BRUISE-CONTROL-2 clinical trial showed that continuous anticoagulation with NOACs (including the morning dose before the procedure) has similar low rates of bleeding complications as the administration of the last dose 48 h before the procedure. Thus, it is considered that depending on the clinical situation and concomitant antiplatelet therapy, both the interruption and continuation of anticoagulants are acceptable [16]. It is important to note that the rate of device pocket haematoma in this study was much lower than predicted and it was underpowered to detect significant differences between strategies. Nevertheless, this is the largest randomized clinical trial evaluating NOACs interruption and continuation strategies during cardiac device implantation.
The peri-procedural NOACs interruption period should be adapted individually considering patient characteristics (including age, stroke risk, history of bleeding complications, concomitant medication, kidney function, etc.) as well as surgical factors [14]. Chronic kidney disease (CKD) is one of the most important comorbidities that determine the choice and dose of anticoagulant as these drugs are predominantly eliminated by the kidneys. Therefore, a dose adjustment and potential periprocedural withdrawal should be based on creatinine clearance (preferably calculated by the Cockcroft-Gault equation) [36]. The most recent ESC guidelines recommend interrupting or continuing NOACs depending on the operator’s choice, and discontinuation should take into account creatinine clearance and type of anticoagulant [8]. According to the EHRA practical guide, when NOACs discontinuation on low-risk procedures is considered, for patients on dabigatran and a CrCl of 50–79 ml/min, it should be discontinued 36 h or more, and for a CrCl of 30–49 ml/min it should be discontinued 48 h or more before surgery. For patients taking an FXa inhibitor and with a CrCl of 15–29 ml/min, the last dose of anticoagulant should be taken 36 h or more before surgery [14].
According to the ESS-PREDI survey, NOACs were discontinued in 86 (88.7%) of 95 patients, and the discontinuation time exceeded 24 h in 53 (61.6%) patients [34]. Thus, there is a clear tendency to choose a discontinuation strategy, but this survey was conducted before the BRUISE-CONTROL-2 study results were published. Although no single strategy is superior in reducing the rate of haematoma, in some clinical situations it may be beneficial to operate without discontinuation of anticoagulants. These could be situations where waiting for the end of the anticoagulant effect may cause unacceptable harm, for example, in patients with complete atrioventricular block and unstable temporary cardiac pacing, or in patients with high CHA2DS2-VASc scores [37–39]. In addition, there is increasing evidence that, in many cases, other electrophysiological procedures, such as atrial fibrillation ablation, could be performed safely without discontinuing NOACs when a standard procedure protocol is followed [40].
Despite the fact that NOACs do not require routine laboratory monitoring because of their predictable pharmacokinetics, in some situations laboratory assessment may be useful. Such situations may be emergencies (serious bleeding, urgent surgery, acute ischaemic stroke) or elective (extremes of bodyweight, renal hypo- or hyperfunction, liver disease, suspected drug-drug interaction, suspected gastrointestinal malabsorption). Available tests include liquid chromatography with tandem mass spectrometry (gold standard for measuring NOAC levels), dilute thrombin time (dTT), ecarin clotting time (ECT) and ecarin chromogenic assay (ECA) for screening for the presence of dabigatran levels, and calibrated chromogenic anti-Xa assays for factor Xa inhibitors, as well as some other screening tests [41]. Although these specific measurements have been proposed and may be considered in high-risk interventions, a ‘time-based’ interruption generally appears safe for the majority of patients and procedures [14].
All reviewed studies observed a very low rate of thromboembolic events after device implantation regardless of anticoagulation strategy, and these findings are consistent with other studies’ results [42]. Therefore, it can be assumed that the frequency of thromboembolic complications is more related to the adequacy of anticoagulation control and not to a certain anticoagulation strategy. Due to the presumably known duration of anticoagulant effect, the use of bridging anticoagulation is not recommended at the time of discontinuation of NOACs, but some studies, albeit in a smaller proportion, employ this strategy. According to a multicentre survey by Deharo et al., bridging was used in 22 of 95 patients (25.6%) receiving NOACs (34). In the studies included in the review, this method was employed in 10% to 13.7% of patients [20, 22, 23]. Thus, in real world conditions, the recommendations are not always followed, but the complication rate still remains low. The results of the reviewed studies also confirm that bridging in patients receiving NOACs during such procedures is unnecessary owing to the very low risk of ischaemic events.
Antiplatelet therapy during implantable cardiac rhythm management device procedures
The use of antiplatelet agents is common among patients requiring device implantation. In the reviewed studies, the incidence of concomitant aspirin use ranged from 10.7% to 52.3%, and P2Y12 inhibitor use ranged from 1.7% to 11.3%. According to the BRUISE CONTROL trial results, concomitant single antiplatelet therapy with anticoagulants was associated with an increased risk of device pocket haematoma [11]. Importantly, antiplatelet use was a risk factor for haematoma, regardless of anticoagulant interruption (odds ratio = 1.965; 95% CI: 1.202–3.213; p = 0.0071) [43]. On the other hand, some previous meta-analyses have shown that single antiplatelet therapy did not increase the risk of haemorrhagic events [28, 44]. Such differences could be due to the fact that previous studies examined the use of antiplatelet agents without anticoagulation therapy. However, the concomitant use of anticoagulants doubles the risk of developing a clinically significant device haematoma; therefore, the European Society of Cardiology recommends temporarily discontinuing the antiplatelet agent during the procedure while continuing the anticoagulant, after assessing the patient-specific risk-benefit ratio [6, 43].
Data from studies investigating the effect of dual antiplatelet therapy (DAPT) on device pocket haematoma formation are controversial. Patients on DAPT have a significantly higher risk of postoperative device pocket haematoma. In a retrospective study Tompkins et al. demonstrated a 4-fold increased risk with aspirin plus clopidogrel compared with a no-antiplatelet control group (7.2% vs. 1.6%, p = 0.004) [45]. Moreover, a meta-analysis of 13 studies by Bernard et al. found a 5-fold increased risk of bleeding with DAPT [44]. Sławek-Szmyt et al. found that dual antiplatelet therapy with clopidogrel as one of the antiplatelet agents increased the risk of bleeding complications 7-fold, and DAPT with ticagrelor as one of the agents increased the risk more than 21-fold [46]. Thus, according to current ESC guidelines, P2Y12 inhibitors should be discontinued 3–7 days (depending on the drug) prior to the procedure whenever possible, depending on the individual risk of thrombosis and bleeding [6]. However, the risk of thrombosis when DAPT is discontinued in the setting of recent coronary stenting is about 29% with mortality from stent thrombosis ranging from 9% to 45% [47–49]. Thus, when a device implantation procedure is elective, it should be postponed until dual antiplatelet therapy is no longer indicated.
Different devices and use of anticoagulants
Implantation of different cardiac rhythm regulating devices has different levels of risk of complications. Sławek-Szmyt et al. demonstrated that implantation of a biventricular pacemaker and implantable cardioverter-defibrillator increased the risk of significant bleeding complications 6-fold [46]. In a meta-analysis by Yang et al., the implantation of these devices also had a 36% higher risk of bleeding compared to pacemaker implantation [28]. Such risk could be explained by the more complex structure and size of the device and the rigidity of the electrodes.
Leadless pacemakers are a state-of-the-art technology designed to reduce the frequency of complications associated with a traditional pacemaker, such as lead displacement or complications related to the device pocket. One of the popular devices of this type is the Micra transcatheter pacing system (Micra TPS). However, there are no evidence-based recommendations for the management of anticoagulants during the period of implantation of these new generation devices. Implantation of a leadless pacemaker into the right ventricle via the femoral vein requires a large venous catheter, and it is unknown whether this may predispose the patient to a higher risk of bleeding, particularly at the vascular access site. According to the results of single cohort studies, the complication rate of leadless pacemaker implantation was low, and the continuous use of anticoagulants during the procedure was considered a safe strategy compared to their interruption [50, 51]. Although larger clinical randomized trials are needed, from the available data it seems that the recommendations for the implantation of traditional devices could be applied to these devices, at least for the time being [52].
The subcutaneous implantable cardioverter-defibrillator (S-ICD) is another innovative device designed to avoid complications related to transvenous electrodes, since it is implanted under the skin without the use of the latter. Small-sample retrospective cohort studies by Evenson et al. and Afzal et al. evaluated the incidence of device pocket haematoma among patients who had warfarin interrupted or continued during the perioperative period of S-ICD implantation. Both studies found that continuous warfarin use was associated with a higher risk of device pocket haematoma (26.1% and 0.04%, p = 0.04; 25% and 1.5%, p = 0.001, respectively) [53, 54]. Thus, the design of the device could also influence the choice of anticoagulation strategy in order to reduce the risk of complications, and the increasing use of these innovative devices in clinical practice may require defined recommendations related to the use of anticoagulants.
Limitations: This study has several limitations. Firstly, this review does not include a meta-analysis. Secondly, the methodological quality of the studies was not assessed. Thirdly, most of the studies included in this review were observational and based on small cohorts or post-hoc analyses of randomized controlled trials. Additionally, most of them were underpowered to detect statistically significant differences. Moreover, due to different safety outcomes and varying, not always standardized definitions of device pocket haematoma, it is rather difficult to generalize the results of the studies. Also, in some of the reviewed studies, there was no prior definition of device pocket haematoma and its subjective assessment was left to the attending physician. Anticoagulant discontinuation periods were also highly variable in the studies and may have influenced the risk of bleeding.
Conclusions
Implantable cardiac device procedures are often performed on patients requiring long-term anticoagulation. It is important to optimize the use of these medications in order to reduce the risk of bleeding and thromboembolism. In patients on warfarin, bridging therapy in the periprocedural period of device implantation is associated with an increased risk of bleeding and is not recommended. The current evidence suggests that both strategies of temporary interruption and continuation of non-vitamin K oral anticoagulants in the perioperative period are feasible and safe. When using anticoagulants and antiplatelets together, during the procedure, antiplatelets should be interrupted depending on the individual risk and benefit assessment. Dual antiplatelet therapy during pacemaker implantation is associated with an increased risk of bleeding and should be avoided as much as possible. Both oral anticoagulant and patient-specific and procedure-specific risk factors for bleeding and thromboembolism should be evaluated in each clinical situation to achieve the most favourable outcomes.








