Introduction
Psoriasis is a chronic inflammatory dermatosis with periods of exacerbation and remission. Psoriasis is characterized by red papules covered with silvery scales on the skin. The prevalence of psoriasis is estimated at 1–3% of the population of Europe and the United States of America [1]. Psoriasis is the result of a complex reaction of many factors, including genetic, environmental and immunological conditions.
It has been shown that the process of atherosclerotic plaque formation is in fact similar to the formation of psoriatic plaque [2]. In both processes, T helper 1 (Th1) lymphocytes are activated, with production of pro-inflammatory cytokines such as tumour necrosis factor α (TNF-α), interferon γ (INF-γ) or interleukin 2 (IL-2). Those cytokines induce and sustain endothelial cell damage [3]. The relationship between psoriasis and atherosclerosis is illustrated by the theory of the “psoriatic march”. According to this theory, systemic inflammation in psoriasis provokes insulin resistance, which in turn causes endothelial cell dysfunction - the first step in development of atherosclerosis and, consequently, myocardial infarction or stroke [4, 5].
A vast majority of authors believe that there is an unquestionable relationship between psoriasis and cardiovascular diseases, which was reflected in the meta-analyses [6–13] compared by Boehncke in a 2018 publication [14].
Material and methods
The study group consisted of 62 Caucasian patients (20 women, 42 men) with confirmed psoriasis vulgaris. Inclusion criteria for the study were: diagnosis of psoriasis vulgaris made at least 1 year prior to the enrolment to the study, informed consent of the patient expressed in writing. Exclusion criteria were: earlier diagnosis of cardiovascular diseases, in particular ischemic heart disease, ischemic stroke or transient ischemic attack (TIA), arterial hypertension, peripheral vascular disease, chronic heart failure and heart rhythm disorders, severe liver or kidney failure, other systemic inflammatory disease, except psoriatic arthritis, use of cardiological drugs, including lipid-lowering drugs.
The control group consisted of 42 healthy volunteers (16 women, 26 men) of the Caucasian race. Inclusion criteria for the control group were: informed consent in writing. Exclusion criteria for the control group were similar to those for the study group, but patients could not have any chronic dermatoses.
The study was approved by the local Bioethics Committee (Protocol No. 615/2013).
Medical history, physical examination and laboratory analysis
The medical history of patients was taken using a questionnaire that allowed collection of the following data: patient’s age at the time of examination, date of diagnosis of psoriasis, coexistent psoriatic arthritis, course of psoriasis – frequency of exacerbations during the year, defined as the number of hospitalizations or non-elective visits to a dermatological clinic, previous treatment of psoriasis, the use of systemic and biological drugs, the use of stimulants, burdened family history of cardiac disorders, understood as premature (in men under 55, in women under 65) occurrence of ischemic heart disease or other cardiovascular diseases, vascular diseases caused by atherosclerosis in family members [15], comorbidities, past diseases, surgery, medications used.
Body weight in kilograms, height in centimetres, waist circumference in centimetres, body mass index (BMI) and blood pressure were measured in all patients.
Venous blood was the material for laboratory tests. Blood was collected form people fasting for previous 12 h. C-reactive protein (CRP), glucose and lipid profile were determined.
Severity of the disease was assessed using the Psoriasis Area and Severity Index (PASI) scale in each patient with psoriasis vulgaris.
Imaging diagnostics
Imaging diagnostics was performed by a specialist in radiology and diagnostic imaging. In order to eliminate the differences in the interpersonal examination technique, ultrasound procedures and the computed tomography assessment were always performed by the same specialist.
Ultrasound examinations were carried out with the use of linear probes emitting ultrasounds at frequencies of 9–12 MHz. Measurements were made in common carotid arteries approximately 2 cm from their bifurcation, on the posterior wall of each artery. The mean value of three manual measurements was calculated for the right side and the left side, respectively.
Cardiac computed tomography examinations were performed without intravenous administration of the contrast agent. Proprietary protocols included in the software of these devices were used to conduct heart examinations. Patients were tested after resting, without the use of medications to slow down the heart rate.
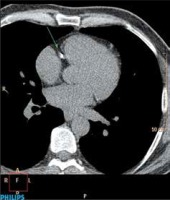
On a Phillips Brilliance Workstation and using HeartBeat CS v. 5.5 software the amount of calcification in coronary arteries was measured. Calculation of this parameter was carried out in a semi-automatic manner. In the first part of the calculations, the software automatically marked a calcification. Coronary calcification was defined as an area of the size of 3 adjacent voxels that were located along the course of the coronary artery and their density was greater than 130 Hounsfield units. Then, after identifying coronary arteries, the study evaluator confirmed that calcifications marked by the software were indeed in those arteries. Then, final results, the Calcium Score according to the Agatston scale (CS), the volume of calcifications in coronary arteries (CV) and the mass of these calcifications (CM) were calculated automatically by the software of the devices (Figure 1).
Statistical analysis
Statistical analysis was performed using the Statistica software [16]. The compliance of the distribution of variables with the normal distribution was analysed with the Shapiro-Wilk test. In 2-group comparisons the Student’s t-test or the Mann-Whitney U test were used. During the research, it turned out that the control group and the study group differed significantly in terms of the frequency of smoking and BMI, therefore, for selected variables, an analysis of covariance (ANCOVA) was additionally performed. It allowed to eliminate the influence of variables affecting research results. Differences between percentages in the groups were estimated with the structure test. A relationship between qualitative variables was tested with the χ2 test with appropriate corrections. The Pearson’s simple correlation coefficient was calculated for the selected variables. In order to assess the influence of selected cardiovascular factors and parameters describing the course of psoriasis on the degree of development of atherosclerotic lesions, multiple regression equations were created. The statistical significance level of p < 0.05 was assumed in calculations.
Results
Characteristics of study groups
Sixty-two patients with psoriasis vulgaris, aged 21 to 68 years (mean: 42.7 years) were qualified for the study. Women constituted 32% of subjects. Each patient included in the study had skin lesions, and severity of the disease as assessed using the PASI scale was on average 14.92 ±6.99 points (M ± SD), with Me = 15.3 points. In the past, systemic treatment was used in 39 people, which constituted 62.9% of the study group. Additionally, 6 patients had coexistent psoriatic arthritis (PsA). People with confirmed psoriasis and coexisting PsA constituted 9.7% of the study group. There were no significant differences between people with psoriasis and those with psoriasis and coexistent PsA in terms of atherosclerosis risk factors.
The 42-person control group consisted of healthy volunteers aged 27 to 67 years (mean: 43.4 years). Women constituted 38% of subjects.
There were no significant differences in mean age, weight, and height between groups (Table 1). The percentage of women and men in both groups was similar. On the other hand, statistically significantly higher average levels of the CRP inflammation index were noted in the study group (Table 1).
Table 1
Characteristics of study groups based on sex (F-women, M-men), age, height, weight, BMI and CRP values of subjects
Assessment of the presence of selected cardiovascular risk factors in the study group of patients with psoriasis vulgaris and in the control group
In terms of the analysed cardiovascular risk factors (hypertension, dyslipidaemia, abnormal fasting glucose, overweight, obesity, nicotine addiction, family history of cardiovascular disease), statistically significant higher BMI values in the study group compared to the control group were noted (Table 1). The mean BMI value in the study group was 26.709 ±4.542 kg/m2, and in the control group the value was significantly lower – 25.031 ±3.339 kg/m2 (p = 0.0323). People with psoriasis also reported smoking significantly more often than subjects in the control group (46.77% of the study group; p = 0.0176).
Assessment of thickness of the intima-media complex (IMT) in patients with psoriasis vulgaris and in the control group
Thickness of the intima-media complex in the study group ranged from 0.600 mm to 1.815 mm and was on average 1.030 ±0.303 mm. The mean thickness of the intima-media complex in the control group ranged from 0.600 mm to 1.400 mm, the mean was 0.838 ±0.151 mm. Differences between groups were statistically significant (p < 0.0001) (Table 2).
Table 2
Mean values of intima-media thickness in the study group and in the control group
Assessment of the amount of calcification in coronary arteries in patients with psoriasis vulgaris and in the control group
In the study group, calcifications were detected in 25 (40.25%) patients, the amount of calcification ranged from 0.2 to 1502 Agatston units (CS), from 0.07 to 283.310 mg (CM) and from 0.1 to 1230.5 mm3 (CV). In the control group, calcification was detected in 10 (23.81%) people, and the amount of calcification ranged from 0.1 to 140 (CS), from 0.04 to 27.34 mg (CM) and from 0.1 to 163.3 mm3 (CV). These differences were statistically significant (Table 3).
Table 3
The amount of calcification in coronary arteries in patients with psoriasis vulgaris and in the control group
Analysis of the relationship between the degree of development of atherosclerotic lesions and the course of psoriasis
On the basis of statistical models, no statistically significant relationship was found between variables describing the severity of psoriasis, such as PASI score, CRP values, duration of the disease or the number of disease exacerbations per year, and the log10 IMT values, the occurrence of coronary calcifications or the log10 CS (Tables 4, 5).
Discussion
The presented study was aimed at demonstrating whether patients suffering from psoriasis vulgaris are at a greater risk of developing atherosclerosis depending on the presence of selected cardiovascular risk factors and the course and severity of the disease.
In our study, patients suffering from psoriasis showed a statistically significantly higher incidence of nicotine addiction. There were also statistically significantly higher values of BMI in the study population.
This is confirmed by other authors. In 1995, Henseler and Christophers proved that most patients with severe psoriasis were overweight [17]. Also, Naldi et al. observed that the increase in BMI was associated with an increased risk of developing psoriasis, and obesity itself doubled the risk [18]. Neimann et al. found obesity in 20.7% of patients with severe psoriasis and 15.8% of patients with mild psoriasis [19].
Both groups also significantly differed in terms of smoking. Neimann et al. found smoking prevalence among patients with severe psoriasis in 30.1%, and among patients with mild psoriasis in 28% [19]. There are also reports that nicotine addiction could affect up to 48.9% of psoriasis patients [20].
In order to demonstrate an increased risk of atherosclerosis, numerous scientific studies use the measurement of thickness of the intima-media complex in carotid arteries. In 2007, Kimhi et al. demonstrated that patients suffering from psoriatic arthritis had higher IMT values compared to the healthy control group [21].
The work of Balci et al. of 2009 was the first publication that showed the thickening of the carotid IM complex in patients with psoriasis compared to the healthy control group (0.609 ±0.146 mm vs. 0.526 ±0.104 mm; p = 0.003) [22]. However, no relationship was found between IMT and PASI values or the duration of the disease.
Subsequently, El-Mongy et al. proved that in the analysed group of patients with psoriasis IMT values were higher than in the control group (0.9 ±0.2 mm vs. 0.7 ±0.1 mm; p < 0.001) [23]. Additionally, it was noticed that thickness of the IM complex was dependent on the patient’s age as well as duration and severity of the disease assessed by the PASI scale.
Subsequent publications confirmed the occurrence of increased IMT values in patients with psoriasis. Those studies differed slightly in terms of patient eligibility criteria, the choice of additional research methods or the analysed data. Arias-Santiago et al. demonstrated an increased number of atherosclerotic plaques in carotid arteries and a thickening of the IM complex in the studied population with severe psoriasis, and this parameter correlated with male sex, severity of the disease on the PASI scale, and duration of the disease [24]. The study population also showed a much more frequent occurrence of the metabolic syndrome and higher values of insulin, aldosterone, homocysteine and inflammatory parameters, such as fibrinogen, D-dimers, CRP and erythrocyte sedimentation rate (ESR). Elsheikh et al. in 2014 also showed higher values of IMT in patients with psoriasis compared to the control group (0.07 ±0.02 vs. 0.05 ±0.01 cm; p = 0.001), especially in the subgroup of patients with a severe form of the disease [25]. Also, Antonucci et al. demonstrated an increased value of IMT in patients with psoriasis compared to the control group (1.465 ±0.5299 vs. 0.89 ±0.26871 mm; p < 0.001) [26]. These authors used strict eligibility criteria, excluding patients with known cardiac risk factors and those taking drugs that could affect the development of atherosclerosis from the study. An interesting study was conducted by Asha et al. [27]. Exclusion criteria included only the use of systemic retinoids or lipid-lowering drugs in the preceding period of 6 months. Therefore, they analysed patients with traditional cardiovascular risk factors, such as obesity, hypertension, dyslipidaemia, type 2 diabetes or nicotine addiction. These authors proved that patients with psoriasis had higher values of apolipoprotein B, apoB/apoA-I ratio and leptin, considered to be unconventional factors increasing the cardiac risk. Also, values of IMT in patients with psoriasis were statistically significantly higher than in the control group. On the other hand, Robati et al. enrolled 40 patients with psoriasis with no cardiovascular risk factors diagnosed on the basis of their medical history for the 2016 study and also showed higher values of IMT compared to the control group [28].
A lot of valuable information on the IMT parameter was provided by the works of Ozturk et al. These authors in their works repeatedly emphasized the fact of the influence of inflammatory diseases on the value of the IMT parameter. They showed some significant differences in IMT values in patients with Behçet’s disease compared to the control group [29]. Also, IMT values in patients with psoriasis were higher than in the control group (0.54 ±0.08 vs. 0.50 ±0.07 mm) [30].
Studies have also shown that thickness of the IM complex closely correlated with inflammatory parameters such as fibrinogen, CRP, high sensitivity CRP (hsCRP), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) and intracellular adhesion molecule 1 (ICAM-1) [30–33].
Martinez-Lopez et al. analysed the effect of systemic drugs and biological preparations on the IMT value [34]. Fifty-three patients underwent carotid ultrasound with IMT assessment and blood serum analysis with lipid profile and carbohydrate assessment prior to systemic treatment. Re-testing was performed 8 months later. A trend towards lower IMT values was found, although noted differences were not statistically significant. Patients treated with ustekinumab and TNF-α inhibitors also had lower glucose and insulin levels.
In the meta-analysis published in 2016, Fang et al. reviewed 20 studies on the subclinical stage of atherosclerosis in patients with psoriasis and in patients with psoriatic arthritis [35]. These authors showed that in majority of studies, patients with psoriasis showed a statistically significant thickening of the IM complex compared to the healthy control group (WMD 0.11 mm; 95% CI: 0.08–0.15).
Another objective method for detecting the subclinical, but also symptomatic stage of atherosclerosis development is the evaluation of the coronary calcification index according to Agatston.
Ludwig et al. were the first to start the stage of research with the use of computed tomography of the heart in patients with psoriasis [36]. In their work, they proved that psoriasis could be an independent risk factor for the development of atherosclerosis and was associated with the presence of a greater number of calcifications in coronary arteries.
In the study by Staniak et al., in which 68% of patients had severe psoriasis, a relationship between the disease and the involvement of coronary arteries was shown, especially with CS > 400 [37]. An interesting study by Hjuler et al. included patients suffering from psoriasis, atopic dermatitis and people from the control group [38]. In this study, the CS value and results of cardiac computed tomography after administration of the contrast agent were used. This enabled identification of both calcified and non-calcified atherosclerotic plaques. Only patients with PASI ≥ 10 were enrolled in the study, and a significantly greater number of patients with CS > 100 was found in the psoriasis group. In this group numerous atherosclerotic plaques and significant coronary stenosis were also found, in contrast to patients with atopic dermatitis who showed atherosclerotic lesions with fewer plaques and stenoses, and lower CS values. Mansouri et al. enrolled patients with psoriasis, type 2 diabetes and healthy controls for the study [39]. In patients with psoriasis and type 2 diabetes, they found a similar profile of cardiovascular risk factors. The prevalence of moderate to severe coronary artery calcification (CS > 100 and CS > 400) was also comparable in both groups and approximately 5 times higher than in the control population. On the other hand, Seremet et al. in their study with the use of cardiac computed tomography did not show a relationship between subclinical atherosclerosis and psoriasis [40].
Coronary arteries on CT were assessed before and after 1 year of treatment by Lerman et al. [41]. These researchers found that patients with psoriasis had a greater volume of non-calcified atherosclerotic plaque and unstable atherosclerotic plaque at high risk of rupture compared to healthy controls. Subsequent cardiac computed tomography examinations after administration of the contrast agent were performed 1 year after the introduction of anti-inflammatory treatment (methotrexate, TNF-α, IL-12/23 and IL-17 inhibitors), showing a reduction in the volume of non-calcified atherosclerotic plaque, thus reducing the overall cardiovascular risk. There was also a correlation between the number of non-calcified atherosclerotic plaques and the severity of the disease on the PASI scale and duration of psoriasis. A similar study was conducted by Hjuler et al. with the assessment of the coronary calcification index in patients with severe psoriasis before and after 13 months of treatment with biological drugs (adalimumab, etanercept, infliximab and ustekinumab) [42]. These authors showed no progression of atherosclerotic lesions in the studied group. On the other hand, an increase in CS values was noted in the control group of patients with psoriasis not treated with biological agents.
In a systematic review and meta-analysis, Kaiser et al., based on the analysis and summary of results of 14 studies involving cardiac computed tomography, showed that patients with psoriasis more often suffered from coronary artery disease, with higher CS values and a greater number of unstable atherosclerotic plaques than the healthy population [43].
The use of at least two imaging methods in scientific research allows assessment of several independent parameters, which enables objectification and strengthening of obtained results. In our study, both thickness of the intima-media complex in common carotid arteries, and the coronary calcification index were assessed in patients with psoriasis, compared to the control group. A similar combined use of IMT and CS assessment in patients with psoriasis was first presented in the 2013 study by Yiu et al. [44]. Based on ultrasound of carotid arteries with the assessment of the IM complex and computed tomography of the heart with the estimation of the amount of calcification, these authors found higher CS values in patients with psoriasis than in the control group (67.4 ±349.2 vs. 0.5 ±3.0, p < 0.05). Also, thickness of the IM complex was higher in the study group (0.73 ±0.11 mm vs. 0.67 ±0.08 mm, p < 0.01). Dowlatshahi et al., in a study in which as many as 76% of enrolled patients had mild psoriasis, showed no significant differences in values of CS and IMT parameters between the group of patients with psoriasis and the control population [45]. Mean values of the coronary calcification index were 53.51 (95% CI: 32.99–86.42) in patients with psoriasis and 55.98 (95% CI: 51.26–61.12) in the reference group (p = 0.86). On the other hand, intima-media thicknesses adjusted for age, gender and cardiovascular risk factors were 1.02 ±0.18 mm in psoriasis patients and 1.02 ±0.16 mm in reference patients, respectively (p = 0.62). Two methods of imaging were also used by Santilli et al. in the paper published in 2015. They showed that 207 patients with diagnosed psoriasis exhibited features indicative of development of early atherosclerotic lesions much more often compared to a 76-person control group [46]. Interestingly, almost half of analysed patients (49%) aged 30 to 39 had changes indicative of early atherosclerosis, compared to 15% in the control group.
It seems important to demonstrate whether the course of psoriasis itself may also affect the degree of development of atherosclerotic lesions. If severity of the disease does play a role in atherogenesis, what tool will allow the best assessment of activity of psoriasis and identify the most at-risk patients.
Studies in murine models confirmed that chronic inflammation of the skin can lead to vasculitis and an increased incidence of thrombosis [47]. Subsequent studies in murine models have shown that it was chronic, not acute dermatitis, which promoted arterial thrombosis [48]. Results of the study by Golden et al. seem to be crucial for further consideration of the role of psoriasis and inflammation in development of atherosclerotic complications. In patients with a longer disease duration, the number of exacerbations and the combined effect of chronic inflammation may be greater and therefore the overall cardiovascular risk may also be increased.
The majority of authors of the works cited so far observed a correlation between IMT results and the PASI score [23, 26, 49] and between CS scores and the PASI score [44]. A similar correlation has not been shown in our study and by several other authors [21, 22, 27, 50]. There was also no correlation for other parameters describing the severity or status of the disease, such as duration of the disease in years, or the number of exacerbations per year, so these data also seem insufficient to fully describe the long-term severity of skin lesions in the course of psoriasis.
Thus, this may suggest that psoriasis increases the patients’ cardiovascular risk, but the role of traditional risk factors appears to be dominant and unassailable. Cardiovascular risk factors play a key role in the development of atherosclerosis and inflammation. Their combination with inflammation present in psoriasis has a synergistic effect and additionally increases the risk of atherosclerosis. Subsequent relapses of the disease and its long course also enhance atherogenesis.
Conclusions
The conducted study showed an increased risk of developing atherosclerosis in patients diagnosed with psoriasis vulgaris. It has been shown that patients with psoriasis vulgaris demonstrate an increased prevalence of cardiovascular risk factors in the form of smoking and excessive body weight. Patients with psoriasis vulgaris show an increased degree of atherosclerosis development compared to the general population, as expressed by higher values of the coronary calcification index (CS) and the thickness of the IMT in carotid arteries. In the population of patients with psoriasis vulgaris, there is no correlation between the degree of development of atherosclerotic lesions and severity of the disease on the PASI scale, CRP value, duration of the disease and the number of exacerbations per year. Patients diagnosed with psoriasis vulgaris constitute a group of people at an increased cardiovascular risk and should be subject to effective screening tests to detect the degree of development of atherosclerotic lesions.