Adequate tissue oxygenation is imperative for survival and requires sufficient levels of haemoglobin (Hb) to transport oxygen. In the case of anaemia, a blood transfusion might be required to ensure adequate oxygen transport capacity. The diagnosis of anaemia is usually confirmed after a blood test [1]. Sampling of blood and analyses of the sample are a time-consuming process requiring a venous or arterial puncture, which may cause patient discomfort and complications (such as haematoma formation and infection) and, more importantly though, it may delay diagnosis of possible life-threatening anaemia resulting in a treatment delay.
In the last decades, percutaneous measurement of Hb has been emerging in the clinical field [1, 2]. A CO-oximetry probe capable of detecting haemoglobin (SpHb) or carbon monoxide and meth-haemoglobin in combination with oxygen saturation was developed by the Masimo cooperation (Radical-7 Pulse CO-Oximeter, Masimo Cooperation, Neuchatel, Switzerland). The main advantage of SpHb measurement is direct display of Hb without requiring time-consuming and expensive blood samples. However, to be a useful decision support tool for transfusion, SpHb needs to be comparable to an invasive measurement. Accuracy of SpHb has been evaluated in a diversity of patient categories with various results. Some studies found a significant correlation between SpHb and invasive Hb, whereas others showed limited comparability [1, 2]. Recently, some influencing factors, such as perfusion index and haemodilution, have been demonstrated by several studies [3–8]. Again, the results are contradictory. Several studies suggested that SpHb could be used as a trend monitor rendering the decision for transfusion of erythrocyte concentrate easier [1, 3, 9–12]. It has even been claimed that using SpHb as a guidance for transfusion decisions would limit the administration of erythrocyte concentrate, resulting in an economic benefit [9, 13, 14]. However, no study has been designed to evaluate the ability of SpHb to give the correct decision whether to transfuse or not.
Therefore, the main objective of this study was to investigate whether using SpHb instead of invasive Hb leads to the same transfusion decisions in patients undergoing major surgery. Secondly we aimed to measure the bias from SpHb from invasive Hb in matched pair analysis.
METHODS
The study protocol was approved by the local medical ethical committee (MEC-2016-044). Signed written consent was obtained from each patient included in this study. Patients were included from August 2015 to December 2017 in Erasmus University Medical Center, Rotterdam, The Netherlands and Maasstad Hospital, Rotterdam, The Netherlands (large secondary care centre). Patients scheduled for surgery with a suspected blood loss of 800 mL or more were included. Type of surgery included hyper-thermic intraperitoneal chemotherapy (HIPEC), liver transplantation, large orthopaedic procedures, open abdominal aortic repair and major gynaecology surgery. Exclusion criteria were atrial fibrillation and age < 18 years. Baseline information such as gender, age, American Society of Anesthesiologists (ASA) classification, transfusion trigger, type of surgery and a baseline Hb was obtained from each patient. The invasive Hb (mmol L-1) was measured with the ABL 90 FLEX PLUS (Radiometer, Copenhagen, Denmark, one machine in each hospital) or in the general clinical chemical laboratory according to the local protocol. Haemoglobin is depicted in mmol L-1. The perfusion index (PI) was obtained to investigate whether SpHb is influenced by perfusion state.
A Masimo Radical-7 Pulse CO-Oximetry sensor K (Masimo Cooperation, Neuchatel, Switzerland) was placed on de 4th finger on the opposite side to the non-invasive blood pressure cuff as per manufacturer recommendations. The sensor was protected from outside light pollution with an optical shield manufactured and provided by Masimo. The SpHb value was not depicted on the monitor; the anaesthesiologist in charge of the patient was therefore blinded to the SpHb reading. Induction and maintenance of anaesthesia were at the discretion of the attending anaesthesiologist. The decision for blood transfusion was based on the national guideline for patients with acute normovolaemic anaemia [15]. This rule is applied in patients with acute normovolaemic anaemia. To summarize: Healthy young patients with acute blood loss will receive erythrocyte concentrate when Hb drops below 4 mmol L–1 (6.4 γ dL–1) or below 5 mmol L–1 (8.0 γ dL–1) if there is continuous blood loss suspected as during major surgery. Patients with extensive co-morbidities, such as coronary heart disease, who are unlikely to compensate for the fall in Hb, will receive blood when Hb falls below 6 mmol L–1 (9.6 γ dL–1).
A pre-operative and post-operative blood sample were drawn from each patient. Depending on the length of the surgery and intraoperative blood loss, additional samples were drawn when clinically indicated by the attending anaesthesiologist. Changes in invasive Hb and SpHb were obtained between intra-operative measurements.
Data extraction (SpHb, PI) was performed by two researchers (AS, RdJ) who were blinded for transfusion data. SpHb was obtained at exactly the same time as the blood sample was taken. Both physicians retrospectively decided the appropriate transfusion trigger by using the extracted data and patient background information [15]. They both separately determined whether a transfusion was indicated. In case of disagreement, they discussed until a consensus was reached.
Outcome parameters
The primary outcome of this study was to investigate whether there would be a difference in the transfusion decision when using SpHb instead of invasive Hb in patients with > 800 mL blood loss during surgery.
Secondary outcomes were the agreement between SpHb and laboratory measurements. The complete dataset was used to compare laboratory Hb with SpHb. Also the influence of PI on accuracy was investigated.
Statistical analysis
IBM SPSS Statistics for Windows, Version 25.0 (Armonk, NY: IBM Corp.) and R statistics 3.5.0 (R foundation, free edition) were used to analyse the data. Continuous, normally distributed variables were reported as means with standard deviation (SD). Non-normally distributed variables are reported as medians with 25th–75th percentile. Bland-Altman analysis, corrected for multiple measurements per subject was used to assess the agreement between SpHb and invasive Hb. The bias (mean difference between the reference and test method) represents the systemic error between the two methods. As a measure of precision (i.e. the spread of repeated measurements), the limits of agreement were calculated as bias ±1.96 SD, defining the range in which 95% of the differences between the methods are expected to lie. Proportional bias was assessed by generalized least squares [16, 17].
RESULTS
A total of 75 patients undergoing surgery with expected blood loss > 800 mL were included in the study. Signal quality was adequate in all patients. In 48 (64%) individuals blood loss exceeded the 800 mL threshold (Figure 1). Demographic data are displayed in Table 1. The average age was 61.6 years, 56% were male and most patients had mild comorbidities (ASA 2, 60.7). Most patients underwent abdominal cancer-related surgery. Nine patients (18.8%) meeting the inclusion criteria (> 800 mL blood loss) received a total combined number of 51 (1–12) erythrocyte concentrates during surgery. Additionally, 44 (0–9) fresh frozen plasma units and 1 unit of thrombocytes were transfused.
TABLE 1
Patient characteristics
Transfusion decision
Retrospectively 13 (27.1%) patients would have received an incorrect transfusion treatment using the SpHb value. In seven patients (14.6%) erythrocyte concentrate would have been given based on SpHb while the invasive Hb did not justify this. Moreover, 6 (12.5%) patients would not have been transfused based on SpHb while they should have based on invasive Hb.
Haemoglobin levels before, during and after surgery
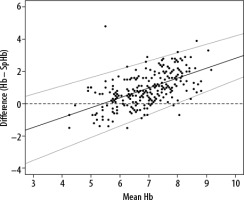
A total of 266 paired measurements of Hb and SpHb measurements were available in 75 patients (2–6 per patient). The mean invasive Hb was 7.44 ± 1.33 mmol L–1 and the mean SpHb was 6.47 ± 0.8 mmol L–1 (P < 0.001). BlandAltman analysis with correction for repeated measurements (Figure 2) revealed a proportional bias with a slope of 0.78 (bias= –4.05 + 0.78 × average Hb). At an average Hb of 5.6 the bias was the smallest. At higher and lower Hb the bias became higher. The lower and upper LOA had the same patterns (bias –1.96 SD = –5.53 +0.68 Hb; bias +1.96 SD = –2.53 + 0.76 Hb).
DISCUSSION
We found that 27.1% of patients would not have been transfused according to the National Guideline (40) using SpHb measurement. Moreover, we found a low agreement between SpHb and invasive Hb (proportional bias = 0.78 × average Hb –4.05).
Our findings correspond with several previous investigations regarding SpHb in the operating theatre and emergency department [7, 18–22]. The Food and Drug Administration approved the Rainbow SET Pulse CO–Oximeter device (Masimo Corporation, Irvine CA) with a published bias of 0.94 γ dL-1 compared with reference laboratory methods in adult volunteers undergoing haemodilution [23]. Therefore, most studies used a cut-off point of 1 γ dL-1, which corresponds to 0.6 mmol L-1. In this study we found a bias larger than that described by Masimo and several other studies [9–11, 24–29].
This difference might be explained by the laboratory method with which invasive Hb was measured. Every hospital has its own gold standard concerning Hb measurement [1, 30]. In our current study, invasive Hb was measured using the ABL 90 FLEX PLUS, which has a bias of 0.08 with small limits of agreement; thus we used a device with good reliability [31]. Therefore, we think the laboratory measurement used does not explain the difference between SpHb and invasive Hb found in our study.
We collected data from several groups of patients including liver transplantation and orthopaedic procedures. In existing literature, there are controversies between studies regarding SpHb studying comparable patient categories. For example, in liver transplant patients Huang et al. found a bias of 2.28 γ dL–1 compared to the results of Erdogan et al. demonstrating a bias of 0.86 [11, 32]. Both studies compared SpHb with Hb measured in a clinical laboratory. It therefore seems that type of surgery cannot explain the difference in bias.
In our current study, no relationship was found between PI and bias between invasive Hb and SpHb. This is in line with previous investigations by Park et al. 6. However, multiple studies revealed a negative relationship between lower PI states and bias of SpHb [3, 5, 33]. It is imaginable that PI could interfere with SpHb, as SpHb is measured in real time in a blood vessel using spectrometry. It might be possible that this study was underpowered to demonstrate a correlation between bias and low PI.
The European guideline for management of perioperative bleeding does not recommend non-invasive haemoglobin devices to target transfusions, due to the bias between invasive and non-invasive measurements. The guideline states, based on previous research, that SpHb could be used as a trend monitor [34]. However, we failed to determine this effect, given that we found evidence of proportional bias with SpHb overestimating Hb at low levels and this difference increasing as invasively determined Hb falls.
Applegate et al. and Gamal et al. observed improving precision of SpHb at lower levels of Hb [23, 35]. In this study precision of SpHb improved until an Hb of 5.6. With a further drop in actual Hb the difference between invasive Hb and SpHb increased. This is particularly disadvantageous at low values because to be useful as a clinical decision tool, high reliability at low values is required given the value of transfusion triggers.
With regard to transfusion of red blood cell concentrate based on transfusion triggers, 27.1% of patients would have received erythrocyte concentrate without justification (14.5%) or did not receive blood when needed according to guidelines (12.5%). Current literature is controversial, with studies describing saving of red blood cell concentrate and even an economic benefit when transfusion decisions are guided by non-invasive measurements [1, 9, 11, 13, 14, 23]. On the other hand, other studies supported our findings. Again, this could be a result of the variability in accuracy between studies.
Limitations
The effect of vasopressors on accuracy of SpHb was not included in this study. Due to technical difficulties the PI was not available in the whole patient cohort.
Due to the study protocol we had no influence in sampling of invasive Hb during surgery; this was only done when clinically indicated by the attending anaesthesiologist. We therefore could not say if the blood sample was taken before or after transfusion. However, it would be logical that transfusion was given after sampling.
The type of surgery is rather heterogeneous.