Introduction
Bowel-associated dermatosis-arthritis syndrome (BADAS) is a rare neutrophilic dermatosis characterized by nonspecific, flu-like symptoms, arthritis and skin lesions. The first information on the association of intestinal bypass surgical procedures with arthritis was first reported in the early 1970s. At that time, it was found that 23% of patients (7 out of 31) developed arthritic symptoms after undergoing jejunocolostomy due to obesity [1]. In 1979, Dicken and Seehafer were the first to observe the occurrence of inflammatory skin lesions, in the form of papules and pustules 2 to 4 mm in diameter, in patients after an intestinal bypass. Due to the initial association of this condition exclusively with bariatric procedures, the syndrome was named “bowel bypass syndrome” [2]. Histopathological examinations revealed features similar to Sweet’s syndrome, classifying this entity as a neutrophilic dermatosis. A few years later, in 1983, very similar symptoms were observed in four patients, none of whom had a history of bowel bypass; moreover, each of them suffered from a different gastrointestinal disease. As a result, it was justified to expand and rename this condition to Bowel-associated dermatosis-arthritis syndrome to include these cases [3]. Nowadays, BADAS has been shown to be associated among others with inflammatory bowel disease (IBD) and small intestine bacterial overgrowth (SIBO) [4]. This narrative review summarizes information about the basics of pathogenesis, clinical manifestations, as well as diagnosis and treatment of BADAS.
Epidemiology
The existing data suggest that BADAS occurs mostly in adults, although cases in children have been reported [5]. The youngest reported patient was the case of a 4-year-old African-American girl diagnosed with ulcerative colitis [6]. Considering the rarity of the disease, there are no data on the prevalence of BADAS by gender; however, it is worth noting that rheumatologic symptoms after intestinal bypass surgery appear to be more frequent in women and in those patients who have undergone jejunoileal bypass surgery [7]. There are reports indicating that in patients who have undergone bariatric surgery, the first symptoms of BADAS usually appear between 3 months and 5 years after gastrointestinal surgery, however a case of onset 18 years after the surgery has been reported [8, 9]. Nevertheless, it is difficult to confirm a causal relationship in such cases. In most patients suffering from BADAS, symptoms and skin lesions without appropriate treatment relapse periodically every 4 to 6 weeks, in a pattern that corresponds to gastrointestinal disease activity [10, 11].
Pathogenesis
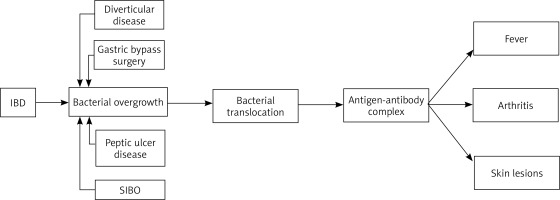
The pathogenesis of BADAS is as yet not thoroughly known. One of the first proposed concepts considered an overgrowth of bacterial flora in the bypassed intestinal fragment as a predisposing factor for this syndrome. In the bypassed intestinal fragment, in addition to the quantitative changes, there are also qualitative changes in the intestinal microflora. In healthy controls, the bacteria of the jejunum are usually aerobic and rarely increase above 105 bacteria/ml. In contrast, over 106 bacteria/ml, both aerobic and anaerobic, have been isolated from bypassed fragments of the jejunum. Bacteroides fragilis is the most common representative of anaerobes, while Escherichia coli dominates among aerobes [7]. However, the concept of bacterial overgrowth had to be expanded after the occurrence of BADAS in non-gastrointestinal bypass surgery patients was described [3]. Among non-bariatric causes, BADAS has been linked to inflammatory bowel diseases such as Crohn’s disease (CD) or ulcerative colitis (UC), peptic ulcer disease, diverticulitis, appendicitis, short bowel syndrome, and SIBO [4, 12–14].
Changes in the anatomical structure of the gastrointestinal tract in various mechanisms can be involved in the promotion of bacterial proliferation. Intestinal diverticula, which most often have a silent clinical presentation, can somehow conceal and protect bacteria in their cavities giving them the opportunity for uncontrolled growth. The consequence of Roux-en-Y gastric bypass (RYGB) is the formation of a blind loop of the intestine, in which bacteria may stagnate and become protected from gastric acid. In inflammatory bowel disease fistulas, narrowings and adhesions may be developed, all promoting abnormal bacterial proliferation. Moreover, impaired function of the ileocecal valve is a well-documented risk factor for SIBO, which is associated with direct retrograde bacterial translocation [15].
Recently, there has also been reported a case of BADAS in patients with cystic fibrosis (CF) [16]. CF is also disease with a high incidence of small intestinal bacterial overgrowth, due to decreased gastric acid production and slowed intestinal motility. Patients with CF are more prone to gastroesophageal reflux and are often prescribed proton pump inhibitors, which are considered a risk factor for the development of SIBO. In addition, CFTR receptor dysfunction in the gastrointestinal tract may cause a thickening of intestinal secretions which can lead to impaired motility and even, in extreme cases, intestinal obstruction [16].
All of the aforementioned conditions disrupt the integrity of the intestinal mucosa, and lead to translocation of intestinal bacteria into the bloodstream [7]. Bacterial translocation is known as the passage of bacteria or their products, such as endotoxins, from the gastrointestinal tract to the mesenteric lymph nodes and systemic circulation. A bacterial passage from the intestinal lumen is common in inflammatory bowel diseases and is involved in their pathogenesis by inducing, maintaining and exacerbating inflammation [17]. Furthermore, it is currently suggested that the overgrowth of gut microbiota leads to increased release and accumulation of toxic bacterial metabolites such as peptidoglycans (PGs). Bowel microbes continuously remove PG from their cell walls as they grow and divide. These molecules are able to pass the intestinal barrier and enter the bloodstream where they interact with the human immune system in many ways. Beyond promoting inflammation, they can also damage the brush border of enterocytes impairing the maintenance of integrity and increase the permeability of the enteric barrier [18, 19]. In the pathogenesis of BADAS, peptidoglycans most likely act as antigenic triggers for an abnormal, generalized immune system response and the formation of immune complexes. The antigen-antibody complexes can induce systemic symptoms such as fever and are deposited in the skin and joints leading to the skin lesions and arthritis which are characteristic of BADAS (Figure 1) [7, 13]. This theory was tested on animal models using bacterial peptidoglycans derived from group A streptococci, which have a similar antigenicity and structure to human bacterial intestinal microflora. They induced BADAS-like skin lesions and arthritis [20].
Clinical presentation
BADAS typically manifests itself in three forms of symptoms: skin changes, arthritis and influenza-like symptoms. The latter present as fever, chills, malaise, and muscle pain. Typically, these flu-like symptoms appear early in the course of the disease and precede any skin changes. Some describe them as similar to sickness serums [10, 21].
In regard to joint manifestations, joint pain in BADAS tends to occur in episodes and is typically asymmetrical, although in some cases it can be symmetrical [22]. It is most common that larger, peripheral joints are involved, e.g. knee, ankle, and elbow, however, cases where arthritis occurred in the metacarpophalangeal and metatarsophalangeal joints have also been reported [9]. The polyarthritis may also be accompanied by tenosynovitis, nevertheless, no long-term damage or deformity had been observed [8, 21, 23].
Inflammatory skin lesions which have a tendency to spread are typically located on the trunk and proximal parts of the upper extremities [5, 22]. In some patients, lesions also appear on the lower extremities, palms as well as soles and even in one case on the face [6, 9, 24]. The typical primary skin lesions in BADAS are red, erythematous macules of 3–10 mm in diameter with indistinct borders. The acute skin lesions may imitate urticaria or insect bites [8]. Over the subsequent 1–2 days, the lesions harden and assume a papulopustular appearance, measuring 2–4 mm in diameter [4]. It should be emphasized that the course of skin lesions in BADAS may be atypical. Erythema nodosum-like lesions and an abscess-forming course of the disease have been described [10, 25]. Chen et al. reported a case of BADAS with large violaceous plaques with grey pseudo-bullae formation surrounding a central eschar with borders of bright red erythema, which occurred on the legs [24]. While, Heard et al. also recorded an atypical case in which symptoms included: vesiculo-pustules and erosions on the lower back and buttocks, eroded vesiculo-pustules in the armpits, and an unusual occurrence of aphthae on the oral mucosa [26]. Although the clinical presentation in children is similar to the classic form of BADAS in adults, unusual presentations have also been reported in the form of scattered haemorrhagic vesicles, or in another recently described case of pink oedematous papules coalescing into plaques across the face with pinpoint pustules scattered on the face, trunk, and upper extremities [5, 6]. Despite skin-related symptoms commonly being the dominant feature, Alshahrani et al. described an instance in which the acute symptoms were limited to the musculoskeletal system. The patient had severe pain with swelling, redness, and limited mobility in the ankle and knee joints, and an ultrasound examination confirmed enthesitis and enthesopathy at the insertion sites of the Achilles tendons [13]. Complications of BADAS include diarrhoea, liver dysfunction, hyperuricemia, calcium oxalate renal calculi, mood changes, tenosynovitis, and vitamin A or B1 deficiency [16].
Diagnostics
Given the unknown aetiology and the diverse clinical presentation of this condition, diagnosis is more than often very challenging, with no established clinical diagnostic criteria for BADAS. The typical form of skin eruptions and the patient’s history, including bariatric surgery or gastrointestinal disease, are in many cases used to establish the diagnosis. The results of routine blood tests are usually normal, although in certain instances markers of inflammation can be found to be increased, including erythrocyte sedimentation rate and C-reactive protein as well as concentration, or the number of white blood cells [6, 9, 13, 25]. Certain supplementary testing may help when confirming or guiding toward the diagnosis of BADAS. Blood microbiological tests are negative for microorganisms (bacteria or fungi) [5]. Tissue cultures and direct immunofluorescence tests are also negative [25].
In certain situations, it is reasonable to perform a hydrogen breath test. This is a non-invasive test that measures the amount of hydrogen and methane one exhales after drinking lactulose. A rapid increase in exhaled hydrogen or methane may indicate bacterial overgrowth in the small intestine and thus help to diagnose BADAS [4, 9]. Imaging tests are not commonly used in BADAS, although there was a report in which a PET scan of a patient with skin lesions and polyarticular pain allowed to narrow the differential diagnosis allowing to confirm BADAS diagnosis. It also allowed for the classification of skin lesions and the selection of a lesion for biopsy [9].
As many case reports have shown, the skin-related symptoms of BADAS can be inconsistent, that is why histological examination of the skin lesions appears to be the most crucial aspect in diagnosis. The typical histological presentation of a skin biopsy shows papillary dermal oedema and a dense, often perivascular, neutrophilic infiltration, which is most commonly confined to the upper dermis. Additionally, leukocytoclasia may also be observed, while fibrinoid vascular degeneration and leukocytoclastic vasculitis are absent [10]. While the infiltration in BADAS is typically neutrophilic, it has been reported that other types of cells, such as lymphocytes, histiocytes, and eosinophils, may also be present, especially in later lesions [5, 11, 24]. With regard to leukocytoclastic vasculitis, there are differing opinions on its occurrence in BADAS. Some deny its presence altogether, while others report that leukocytoclasia may be observed, but without primary vasculitis [22, 26]. The literature is consistent on the absence of fibrinoid vascular degeneration in the course of BADAS, although a case has been described where fibrinoid necrosis was demonstrated in the histopathological image [9]. Skin changes in the course of BADAS can be diverse, which can cause the disease to resemble several other dermatoses, such as pyoderma gangrenosum, hidradenitis suppurativa, panniculitis, septic abscess syndrome, and Sweet’s syndrome [12, 25]. Therefore, obtaining a thorough medical history regarding factors that induce the development of this disease, identifying the presence of joint symptoms, and performing a skin biopsy is pivotal.
Treatment
Since the condition is rare, there are no standard recommendations for BADAS treatment. In some cases, the changes spontaneously resolve, however, in some patients, pharmacotherapy is required (Table 1). The therapeutic management targets the key elements of the pathophysiology of this condition, i.e. bacterial overgrowth, neutrophilic inflammation and underlying disease that leads to the translocation of bacteria from the intestines to the peripheral circulation.
Table 1
Treatment modalities of bowel-associated dermatosis-arthritis syndrome (BADAS)
| Authors | Gender and age | Treatment | Effects and recurrence | Medical history |
|---|---|---|---|---|
| Heard et al. [26] | Female, 49 years old | Ustekinumab therapy was commenced with a saturating dose, 260 mg intravenously. The patient was discharged from the hospital with a maintaining dose of 90 mg administered subcutaneously every 8 weeks. | Significant improvement in cutaneous and oral lesions at 2-week follow-up and complete resolution of lesions at 3-month follow-up as well as improvement in gastrointestinal symptoms. No information reported on the occurrence of relapses. | Crohn’s disease Gastroesophageal reflux disease Mitral valve leaflet prolapse Stroke Anxiety Depression |
| Rosen et al. [16] | Female, 47 years old | Oxacillin (2 g/day) and Ceftolozane/tazobactam (4.5 g/day) for several days throughout hospitalization. Additional continuation of treatment for 2 weeks after hospital discharge. | Rapid improvement in joint pain and skin lesions. No information on the presence of relapses. | Cystic fibrosis |
| Ashok, Kiely [23] | Female, 23 years old | 60 mg prednisolone/day | Fast response to the administered drug; no recurrences for 3 years. | Crohn’s disease, resistant to immunosuppressive drugs, which included azathioprine, corticosteroids and three infusions of infliximab. A month before her admission, a laparotomy was performed for excision of a complex fistula. Fixed inoperable ileostomy due to persistent abdominal pain (2 weeks prior to admission). |
| Zhao et al. [4] | Male, 29 years old | Doxycycline 0.2 g/day for a week along with Methylprednisolone 40 mg/day for a week | Significant improvement overall. After a 12-month follow-up, there was no recurrence. | Reactive arthritis, use of NSAIDs (celecoxib) caused resolution of symptoms for a brief period of time. No history of gastrointestinal surgery or inflammatory bowel disease. |
| Richarz et al. [29] | Female, 51 years old | Ciprofloxacin 500 mg twice a day for 14 days and Trimethoprim/sulfamethoxazole 600/120 mg twice a day for 7 days. | After treatment, the symptoms resolved, and the patient remained asymptomatic beyond 6 months. | Bariatric surgery in 1993 (Salmon technique) |
| Chen et al. [24] | Male, in his 40s | Doxycycline – oral administration at a dose of 100 mg twice a day, treatment of long duration. Additionally, collagenase poultices on the lesion. | The skin lesions and pain symptoms resolved, and there is no information on recurrence. | Two months prior to the admission, the patient was hospitalized for a cholecystectomy that was complicated by a postoperative abscess and duodenal perforation. |
| Barland et al. [33] | Male, 39 years old | Metronidazole and fluoroquinolone, followed by prednisolone 0.5 mg/kg. | Nearly complete remission within 4 weeks following initiation of therapy. No information on the occurrence of relapses. | Ulcerative colitis induction – treated with methylprednisolone. As maintenance treatment, a cycle of prednisone with a gradual dose reduction plan was used, in addition, mesalamine and azathioprine were applied (complete remission of the disease was not achieved). |
| Oldfield et al. [6] | Female, 4 years old | An 11 day admistriation of metronidazole and prednisone. Subsequent infusions of infliximab every 8 weeks. | Symptoms resolved, and no subsequent relapses of the syndrome were noted. | Ulcerative colitis Autoimmune hepatitis Sickle cell anemia |
| Havele et al. [5] | Male, 6 years old | Local steroids (triamcinolone) were applied. Subtotal colectomy was performed followed by treatment with ruxolitinib. | The patient responded to treatment, no information on recurrence. | Very early onset inflammatory bowel disease (refractory to treatment by: infliximab, vedolizumab, canakinumab, antibiotics, mesalamine and steroids). |
| Johns et al. [32] | Female, 14 years old | Two doses of methylprednisolone 40 mg each (0.6 mg/kg) were administered, followed by a dose reduction and the addition of sulfasalazine. After the second hospitalization, sulfasalazine and infliximab were commenced. | Rapid improvement of both skin symptoms and the gastrointestinal and joint symptoms. Recurrence of gastrointestinal symptoms after several months, followed by good control of symptoms as a result of the included treatment with sulfasalazine and infliximab. | Ulcerative colitis |
Antibiotic therapy is a remedy for excessive growth of the intestinal flora, thus this form of treatment prevents the formation of circulating immune complexes that are suspected to be the cause of systemic symptoms [26–28]. It has been reported that antibiotic therapy is particularly effective in patients with BADAS caused by SIBO and CF [4, 16]. In addition, a significant improvement in joint pain has been noted in patients treated with antibiotics [16]. Tetracycline, erythromycin, clindamycin, sulfamethoxazole, trimethoprim or metronidazole therapies have been successfully conducted, but relief after their use was usually only temporary and varied between 6 and 12 months [4, 9, 24, 29]. The preferred form is oral antibiotic therapy, but it should be emphasized that it is necessary to continue the therapy even once the patient’s condition has stabilized [16, 24]. Effective antibiotic therapy causes the skin changes to subside slowly, preventing the development of new lesions. Erythematous papules and pustules have been reported to resolve, it was also noted that itching was relieved and joint pain improved radically [16, 24]. To prevent recurrence, rotation of antibiotics or prokinetic agents, such as cisapride, which support cleansing the small intestine may be useful [30, 31]. For paediatric patients, the choice of antibiotics depends on age-related contraindications [22].
Administration of steroid therapy (local and systemic) is used to reduce the excessive inflammatory reaction. NSAIDs and immunosuppressants (cyclosporine, mycophenolate mofetil) can be administered to reduce the steroid dose. Dapsone administration is another treatment alternative [24, 29]. The efficacy of the treatment is considered good, and results in a rapid improvement of patients’ skin, gastrointestinal and joint symptoms [9, 32]. Steroid therapy and immunosuppressive therapy are particularly effective in the treatment of BADAS caused by IBD, and remission can last up to 3 years [23].
In these patients, oral canker sores may “precede, coexist, and/or reflect inflammatory bowel activity”, and observation of lesions and early treatment may help prevent recurrence [33]. Some patients with IBD benefit greatly from combining steroid therapy with antibiotic therapy, and their condition improves in a short period of time with such treatment [33].
It is worth considering the inclusion of biological treatment, especially in the case of disease recurrence or after ineffective antibiotic/steroid therapy, as well as in paediatric patients in whom BADAS could be the first symptom of IBD [5, 6, 26, 32]. In the treatment of this disease, interleukin-12 and interleukin-23 inhibitors, ustekinumab, have gained prominence in recent years [26, 34]. Most reports refer to the use of this drug in the treatment of neutrophilic dermatoses, most often pyoderma gangrenosum associated with Crohn’s disease. Heard et al. applied this treatment and reported a complete remission of Crohn’s disease in their patients, of which 4 out of 7 had neutrophilic dermatosis [26]. They observed an improvement not only in skin lesions, but also in changes of the oral cavity, as well as gastrointestinal symptoms reduction [26, 35, 36]. Recurrences after successful biological treatment are uncommon [6]. Infusions of infliximab (TNFα-inhibitor) and ruxolitinib (Janus kinase inhibitor-JAK) also seem to be effective [5, 6].
Surgical treatment of BADAS is a known procedure since the condition was originally thought to be caused by bypass surgery only. Restoration of the normal anatomy of the intestine by means of another surgical operation, eliminating the intestinal loop, brought recovery in many cases [37]. In turn, there have also been cases in which a subtotal colectomy was necessary due to extensive involvement of the intestine [5]. In addition, the appropriate local therapy for skin lesions should be remembered [26, 38].
Conclusions
It is currently assumed that the pathogenesis of BADAS is related to conditions that disrupt the integrity of the intestinal mucosa, leading to the translocation of intestinal bacteria. Due to the diverse clinical presentation that mimics other neutrophilic diseases, for years this disease has been mishandled and consequently underdiagnosed. The histopathological examination combined with the clinical course and patient history remains the most essential in the diagnosis, however, it is worth highlighting the increasing role of other diagnostic methods such as the hydrogen breath test. While often the disease can resolve spontaneously, at times it is necessary to resort to treatment, with antibiotic or immunosuppressive drug therapy targeting the key elements of the disease’s pathogenesis, i.e. bacterial overgrowth and immune response. The application of biological treatment in this disease is of great interest since it ensures a long remission in patients with multiple relapses.