Purpose
Brachytherapy is an essential component of uterine cervical cancer treatment [1]. In image-guided brachy-therapy (IGBT), target and surrounding organs, such as the bladder, rectum, and sigmoid, can be contoured, and their volumes and dose distribution can be determined. Dose distribution can be optimized by adjusting dwelling weight and dwelling position of the radiotherapy source inside the applicator, to ensure that the optimal dose is delivered to the target while limiting dose delivered to surrounding organs. Consequently, IGBT can improve local control, overall survival rate, and reduce toxicities [2-7].
The reported dose of IGBT to the target and organs at risk (OARs) is established as calculated dose determined from treatment planning; however, delivered dose can be different from calculated dose. While dose differences can result from a range of uncertainties, intra- and inter-fraction uncertainties account for 11% and 20-25% in doses delivered to target and OARs, respectively [8]. This accounts for major portion of the total uncertainty budget. Although contouring is the main factor in the intra- and inter-fraction uncertainties of the target (9% of the 11%), it accounts for only 5-11% of the total 21-26% uncertainty budget for OAR doses, while the remaining major portion occurs as a result of organ motion [8].
After applicator insertion and volumetric image acquisition for IGBT, certain processes that create a time gap before brachytherapy delivery, such as target and OARs delineation, applicator reconstruction, and treatment planning, may lead to organ motion or applicator displacement. Displacement of tandem and ring by more than ±3 mm could cause uncertainty of more than 10% in both conventional (point A) and 3D image-guided brachytherapy [9]. Organ motion can contribute to significant degrees of uncertainty in doses delivered to OARs [10].
The use of in-room volumetric image-guided bra-chy-therapy has grown in recent years [9, 11]. Our center has installed and used in-room computed tomography (CT)-based brachytherapy since January, 2019. After insertion of brachytherapy applicators, CT image and brachytherapy delivery can be achieved in the loading room without having to move the patient. Although this system has the potential to limit patient’s movement, this system can also limit utilization of the brachytherapy loading room, and thus can affect a high workload center, such as ours.
Switching patients out from CT table during planning process and then transferring them back for brachytherapy delivery, even though CT machine is located within the brachytherapy loading room, can support the overall period of the treatment. This is similar to centers, in which the image machine is outside brachytherapy room (referred to as ‘out-room’ brachytherapy). Therefore, we can deliver brachytherapy to one patient while conducting parallel treatment planning for another. However, the movement of patients can also lead to applicator displacement and organ motion.
In this study, we compared the changes in dose and volume of OARs from CT image immediately before brachytherapy delivery to CT image for treatment planning between in-room brachytherapy (IRBT) and out-room brachytherapy (ORBT). Because of the limitations of CT image in terms of assessing tumor extension, we did not assess the changes in doses to the target.
Material and methods
This was a prospective randomized unblinded study. Eligibility criteria were pathologically proven non-metastatic cervical cancer patients, within an age range of 17 to 70 years, ECOG performance status of 0-2, FIGO stage IA1 to IVA, and treated with a definitive combined treatment of external beam radiotherapy and brachytherapy with or without chemotherapy. Exclusion criteria were prior abdominal surgery, previous chemotherapy, earlier pelvic radiotherapy or brachytherapy, and patients who were currently pregnant or breast-feeding.
External beam radiotherapy
Forty-five to forty-six Grey (Gy) of whole pelvic radiotherapy (WPRT) was delivered with an additional 10-17.5 Gy boost to enlarged lymph nodes via simultaneous integrated boost or sequential boost. Treatment was delivered with conventional radiotherapy or intensity-modulated radiation therapy (IMRT). Additionally, forty milligrams per square meter of weekly cisplatin or AUC2 of weekly carboplatin were concurrently administered to patients with locally advanced disease.
Brachytherapy
Computed tomography-based image-guided brachy-therapy was initiated at the final week of external beam radiotherapy, with a dose of 28 Gy in four fractions. Applicator insertion, image acquisition, and treatment planning were doe for each individual fraction. Each fraction was delivered after at least two days from the previous fraction. Cumulative dose of external beam radiotherapy and brachytherapy to high-risk clinical target volume (HR-CTV) was at least 80 Gy in EQD2. Applicators were either tandem with ovoid, tandem with ring, or tandem with cylinder, based on tumor extension. Interstitial needles were inserted in patients with extensive tumor extension or those with difficult anatomy.
Patients were randomized by computer-generated code using random block sizes of 4 with 1 : 1 allocation at the time of first fraction of brachytherapy into two arms (IRBT and ORBT).
During each brachytherapy fraction, Foley’s catheter was inserted and set on free flow until no residual urine was present. Patients were also advised to consume a soft diet during WPRT and brachytherapy; however, no particular bowel preparation was applied during the course of brachytherapy. The applicator was applied in the operating room, and the patient was then transferred to CT machine located in the brachytherapy loading room. Previously empty urinary bladders were then filled with a hundred milliliters of contrast solution that was diluted in normal saline solution (NSS). After that, a CT image without intravenous contrast (CT1) was acquired from the sacroiliac joint to the lesser trochanter, with a thickness of 3 mm per slice, at which point, NSS in the bladder was released. Patients who were receiving treatment in the IRBT arm remained on CT table in the loading room while waiting for treatment planning. Alternatively, patients receiving treatment in the ORBT arm were transferred to the waiting room. This was done to mimic image-guided brachytherapy that was delivered in the center, with no volumetric image machine inside the brachytherapy loading room where the patient would need to be moved between the brachytherapy unit and the image machine unit.
HR-CTV was contoured based on contouring guidelines established by Viswanathan et al. [12]. Whole volume of the bladder, rectum, and sigmoid were contoured as OARs in all patients. Oncentra brachytherapy treatment planning system version 4.5.3 with TG-43 algorithm was applied for contouring and treatment planning.
After brachytherapy plan approval, patients in the ORBT arm were transferred back to the brachytherapy loading room. Subsequently, patients in the ORBT arm underwent three transfers (from applicator insertion to CT table, then were moved to the waiting room, and back to CT table in the loading room) in total, while patients in the IRBT arm underwent only one transfer (from applicator insertion to CT table).
The bladder was then once again filled with one hundred milliliters of NSS to ensure the same volume of the bladder filling as before, and a second CT image (CT2) was acquired for both the IRBT and ORBT arms’ patients immediately prior to brachytherapy treatment delivery. Before treatment delivery, any visualized changes or displacement were corrected by the physician.
The treatment arm switched after each fraction of brachytherapy. Patients who were in the IRBT group in the first fraction were switched to the ORBT group in the next session, and to the IRBT and ORBT groups in the 3rd and 4th fractions, respectively. Therefore, each patient received two IRBT and two ORBT fractions and treatment plans. Randomization during the first fraction and switching during subsequent fractions were applied to limit the effects of tumor shrinkage, which can occur during the subsequent brachytherapy session on treatment planning between arms.
Brachytherapy doses of CT1/CT2 and dose/volume comparisons
CT2 images were co-registered with CT1 images using rigid registration of the applicator. In the registration process, at least three points on applicators, including one point at the tip of the tandem, at least two points at the uppermost point of the ovoid/ring/cylinder, and at an additional point(s) along the needles if they were used, were positioned in both CT1 and CT2 images. Registered images were verified to confirm the same position of applicator.
Additional contouring of OARs on CT2 was performed, assuming the same shape and volume of HR-CTV in both CT1 and CT2 images. As a result, two contours of the rectum, bladder, and sigmoid were observed: the first contours were obtained from CT1, while the second ones were obtained from CT2. Accordingly, D2cc doses (minimum dose in the most exposed 2 cm3 volume) and volumes of the bladder, rectum, and sigmoid from both CT1 and CT2 images obtained from brachytherapy treatment planning on CT1 were recorded. Differences of D2cc and the volume of OARs were then calculated.
This study was granted approval by ethical committee of Faculty of Medicine, Chaing Mai University, approval No. 030/2020; and registered with the Thai Clinical Trials Registry ID TCTR20200605008. This work was supported by Faculty of Medicine Research Fund, Faculty of Medicine, Chiang Mai University, grant no. 111-2563.
Statistical analysis
Based on a study conducted by Anderson et al. [13], dose change in comparisons made between planning MRI and pre-treatment MRI for the bladder was 0.5 ±0.5 Gy. We assumed that dose change in the IRBT system would be 35% lower. With a power of 80% and an alpha value of 0.05, we calculated total fractionations of BT in both the arms as 256.
Patients and brachytherapy characteristics were presented using descriptive statistics as mean with standard deviation for quantitative data, and as numbers with percentages for categorical data. Mean values of volume and dose of OARs between CT1 and CT2 were compared using Wilcoxon signed-rank test. A comparison of changes of these parameters between the two methods for the IRBT and ORBT groups was performed using Mann-Whitney U-test. Relationship between dose difference and volume difference in each organ was analyzed using Spearman’s rank correlation. Accordingly, results were statistically significant if p < 0.05. Data were evaluated by STATA software version 16 (Stata Corp. LLC, Texas, USA).
Results
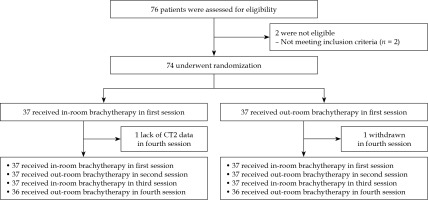
Seventy-six patients were assessed in terms of eligibility. Accordingly, 74 patients with 274 (137 IRBT and 137 ORBT) fractions of brachytherapy were included in this study. The fourth brachytherapy session data was not available for two patients due to withdrawal after treatment and a lack of CT2 data (Fig. 1). The most common applicator was tandem and ovoid. Hybrid intra-cavitary and interstitial brachytherapy were used in 25.17% of cases. In this study, there was no applicator displacement observed by visual inspection between CT1 and CT2 on any of the fractions. Relevant data relating to patient characteristics, applicator types, percent of hybrid brachytherapy, and mean time between CT1 and CT2 images are presented in Tables 1 and 2.
Table 1
Patient characteristics
Table 2
Brachytherapy characteristics
According to comparisons of the volumes and doses between CT1 (planning CT) and CT2 (pre-delivery CT), the results indicated that the changes in volumes were statistically significantly different for both the IRBT and ORBT arms, with the exception of the volume of the bladder in the ORBT arm. The mean CT1 vs. the mean CT2 volumes (ml) for the bladder, rectum, and sigmoid (mean ±SD) in the IRBT arm were 185.89 ±59.22 vs. 175.43 ±59.16, p = 0.0294; 39.85 ±22.02 vs. 37.53 ±22.29, p < 0.001; and 63.73 ±39.94 vs. 57.53 ±34.08, p < 0.001, respectively; and in the ORBT arm were 170.80 ±39.38 vs. 176.94 ±58.83, p = 0.2261; 41.88 ±19.73 vs. 39.64 ±18.64, p < 0.001; and 57.69 ±33.63 vs. 52.48 ±27.72, p < 0.001, respectively.
The mean D2cc doses to OARs were also significantly different in comparisons between CT1 and CT2, except for D2cc doses to the bladder in the ORBT arm (Table 3).
Table 3
Mean D2cc of organs at risk
However, in comparisons of the mean absolute differences in terms of volume and doses (CT2 minus CT1) between the IRBT and ORBT arms, the data indicated that the mean absolute difference in volume of OARs were not statistically different between the IRBT arm and the ORBT arm, except for the bladder. However, the mean absolute differences in D2cc doses for all OARs showed no significant differences (Fig. 2 and Table 4).
Fig. 2
Histogram of D2cc changes in organs at risk. There were no significant differences in mean dose changes in comparisons made between in-room and out-room brachytherapy
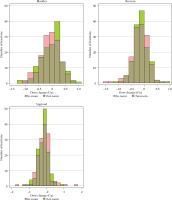
Table 4
Comparisons of volume and dose changes
In the sub-group analysis, comparisons were made for the dose change and type of applicator used. Accordingly, dose change was found to be significantly different between the IRBT and ORBT patients for D2cc of the bladder when using the tandem and ring. Particularly, there were no significant differences for other applicator types or for the remaining OARs. Furthermore, there were no differences in terms of the dose change between hybrid IC/IS brachytherapy and IC brachytherapy alone, and no differences were observed with regard to the amount of time required for brachytherapy (Supplemental Tables S1-S3).
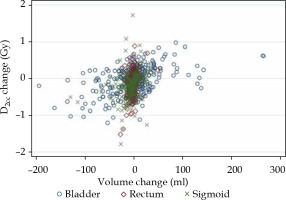
Significant correlations were observed between changes in the volume and changes in D2cc dose delivered to each OAR (Fig. 3). Spearman’s rank correlation coefficient of each organ is shown in Supplemental Table S4.
Discussion
In this study, we evaluated the volume and D2cc dose changes for OARs from CT1 to CT2, and compared between IRBT and ORBT. Because organ motion and applicator displacement is a major part associated with dose changes for OARs [8], our study intended to evaluate the impact of IRBT on these two components. Due to the integration of imaging, planning, and treatment delivery being administered at the same location, IRBT has the potential to reduce patient movement, limit organ motion, and diminish the chances of applicator displacement during the process of transfer.
Changes in the doses to OARs between treatment planning and imaging immediately before brachytherapy have been evaluated in many studies [10, 13-15]. In our study, D2cc doses for OARs showed significant differences between CT1 (planning CT) and CT2 (pre-delivery CT) for both the CT-based IRBT and ORBT, with the exception of the bladder in the ORBT arm. These outcomes are inconsistent with the findings of two previous studies, which reported no significant changes in D2cc doses to OARs. Lang et al. [14] evaluated the uncertainty of doses to the target and OARs of two fractions of MRI-based brachytherapy in the same applicator insertion. An MRI was evaluated in each fraction that was done 6-10 hours apart, and the results revealed no significant differences in doses between these two fractions. Anderson et al. [13], who evaluated changes in the volume and dose (D2cc) of OARs in MRI-based brachytherapy, reported no statistically significant differences in both volume and dose changes of OARs between planning and pre-treatment MRI. Even though non-significant changes were observed in the dose and volume of OARs, these two studies [13, 14] reported greater differences in OARs’ volumes and doses between planning image and pre-delivery image than in the present study. These outcomes could be from the differences in the duration between the two images in comparisons made between our and previous studies. The mean duration times in the present study were 40.33 ±17.21 and 50.42 ±23.97 minutes in the IRBT and ORBT arms, respectively, while the duration in a Lang et al. study was 6-10 hours, and in Anderson et al. study, it was 4.75-10 hours. Conversely, our findings correlated with Nesvacil et al. [10], who demonstrated intra-application uncertainty, resulting in 11% and 20-25% degrees of uncertainty in the dose delivery to the target and OARs, respectively. Even with smaller changes in volumes and doses, we employed a relatively large sample size when compared to the two previous studies (137 in each arm vs. 36 and 84 fractions) [13, 14], with small differences in certain values observed in our study.
By comparing IRBT and ORBT, volume changes in the bladder were statistically significantly different between the two arms, although we used the same amount of saline to fill the bladder in both instances. Even though we did not record the time between bladder filling and image acquisition, we believe that the time could have an impact upon this difference since the additional time can lead to more urine excretion into the bladder. However, the differences in volume changes did not affect the dose changes, as no significant differences were observed in dose changes for D2cc to all OARs.
Although IRBT can limit the movement of patients when transferring them from brachytherapy theater to image acquisition room and to treatment delivery room (ORBT), our findings demonstrate no benefit of IRBT in limiting the dose change between treatment planning and dose delivery. These results were also found to be consistent with the sub-group analysis. Interstitial brachytherapy insertion, time between CT1 and CT2 imaging as well as applicator types, except for tandem and ring, did not reveal any statistically significant differences. Our assumption is that the significant dose change associated with the tandem and ring may have resulted from the characteristics of the ring applicator that have relatively rigid geometry to tandem compared with the ovoid. The applicator would then extend near the bladder. Further studies are needed to evaluate this condition.
Our results also demonstrate correlations in changes in the volume and changes in the doses being delivered to OARs. Consequently, the effort to limit volume changes during treatment should be considered. Our protocol to fill the same amount of NSS in the bladder can limit the volume change in patients in the ORBT group; however, a significant change was observed in the IRBT group. Hence, an adjustment to the bladder filling protocol should be considered in order to minimize the amount of time needed for the bladder to be filled and the image acquisition/treatment. For the rectum and sigmoid, the volume change can occur from peristalsis and affect contents of the bowels, as can the administration of anti-spasmodic medication, bowel preparation, or rectal gas removal. Therefore, appropriate preparation of the bowel should be considered before the procedure is initiated [16-20].
In-room brachytherapy limits utilization of a loading machine in the loading room as patients wait inside the room during treatment planning process. ORBT does not increase the time of the procedure as demonstrated in our results, in which no differences were observed in CT1 to CT2 time interval between IRBT and ORBT. Additionally, more than one patient can be prepared in parallel because the ORBT workflow can free-up the brachytherapy loading room for delivery of treatment, while treatment planning is performed for another patient. As our center experiences a high workload of brachytherapy treatment, ORBT can increase utilization of the loading room without compromising the dose change to patient.
To our knowledge, this is the first study that compared dose change in patients receiving IRBT and ORBT. The number of brachytherapy sessions in our study was relatively large, and included 274 brachytherapy fractions. However, this study faced a number of limitations. Firstly, we used only CT images in our study, wherein GTV dose and changes in HR-CTV volume could not be evaluated due to limitations of CT image on soft tissue resolution when compared to MRI image. Secondly, our study evaluated only volume and dose changes, while all other potential benefits of IRBT, such as patient’s comfort and assessment of the applicators’ position applied during the procedure, should be considered. Thirdly, the results of our study were deemed to be valid for CT-based image-guided brachytherapy, and further studies should be performed involving MRI-based image-guided brachytherapy. This is because a longer time interval between imaging and brachytherapy delivery could result in greater dose changes.
In conclusion, the outcomes of our study indicate that IRBT does not result in a significant difference in the dose change between planning and pre-treatment imaging when compared to ORBT. Consequently, ORBT can be considered for routine practice in high workload centers.