Introduction
Short-acting β2-agonists in inhalation (SABA) such as salbutamol (albuterol, levobuterol), fenoterol and terbutaline have been standard bronchodilator drugs used as rescue medication in asthma and other chronic or acute obstructive pulmonary diseases [1–4]. SABA is also used in bronchodilator response testing (BDRT), which is an important diagnostic tool in bronchial obstructive diseases in both children and adults [1, 2, 5, 6]. Salbutamol is available in a pressurized metered-dose inhaler (pMDI), a breath-actuated metered-dose inhaler (pMDI-BA), different types of dry powder inhalers (DPIs), a metered-dose liquid inhaler (MDLI) and as a nebulization solution [7, 8]. This drug in BDRT is administered most often with pMDI alone or via suitable valved holding chamber (VHC) or sometimes from nebulizers [5, 9–11].
The bronchodilator response (BDR) of SABA depends on many factors, including a deposited dose in the lower airways [12], polymorphism of the β2-adrenergic receptors [13–15], the initial degree of obstruction of bronchi and bronchioles, and the cause and mechanism of their obturation [5]. The dose of salbutamol deposited in the tracheobronchial tree depends on the characteristics of the inhaled cloud and how the patient is breathing [16]. The characterization of the aerosol cloud of each nebulized drug is influenced by the aerosol generation method (jet/pneumatic vs. ultrasound nebulizer), in particular the characteristics of the nebulizer head, and in the case of jet nebulizers, also the type of compressor [8, 17]. Technical differences between nebulizers cause that the dose of medicine leaving different types of these devices may differ from each other even more than 10 times [18]. In the case of jet nebulizers, another element which can influence the clinical effectiveness of the drug is the method and technique of nebulization: continuous nebulizer vs. breath-actuated nebulizer vs. nebulizer adapting to the breathing pattern [7, 19–21].
There are relatively few studies comparing the clinical effects of the same drug inhaled from various nebulizers, especially in children [22–30]. This is due to the methodological difficulties of such studies (the technological diversity of nebulizers, calculation of drug doses) and the lack of legal regulations from the FDA or EMA that may be the basis for such comparisons [21, 31, 32].
Aim
The main goal of the study was the spirometric evaluation of the BDR of two methods of salbutamol nebulization in asthmatic children with bronchial obstruction. The additional goal was to assess the tolerance of the inhaled drug.
Material and methods
Study design
A single-site, randomized, open, comparative study was conducted in the Pulmonology Outpatient Clinic at the University Children’s Hospital (a tertiary care hospital) in Lublin (Poland) between January 2010 and May 2017. This study was approved by the Local Bioethics Committee of the Medical University of Lublin (Resolution No. KE-0254/121/2009) and performed in accordance with the Declaration of Helsinki. All participants and their parents provided written informed consent.
The primary study objective was to assess the change in forced expiratory volume in the first second (FEV1) and forced expiratory flow at 25–75% of vital capacity (FEF25–75) values 15 min after salbutamol administration using two different nebulization methods vs. baseline values in children with bronchial obstruction. The secondary objective was to assess the prevalence of typical adverse effects of SABA, such as tremors, vomiting, palpitation, increase in blood pressure and heart rate during 1 h of observation.
Patients
Eligible participants were children aged 6 to 17 years with partially controlled asthma, recognized by the physician at least 6 months before the study and treated according to GINA recommendations [33]. The other inclusion criteria included: ability to perform correct spirometry (at least once correctly performed spirometry within 12 months before inclusion in the study), ability to perform correct nebulization with the mouthpiece (previous experience with nebulization), bronchial obstruction determined by spirometry (percent predicted FEV1 (FEV1%) < 80%). Key exclusion criteria were as follows: features of respiratory tract infection in the last 4 weeks before the study, use of SABA in the last 6 h, ipratropium bromide in the last 8 h, long-acting β2-agonists (LABA) in the last day, tiotropium bromide in the last 7 days, systemic corticosteroid in the last 30 days before the study, passive and active tobacco smoking, FEV1% ≤ 50% [34].
The sample size was calculated based on a similar study in children, in which a group of 72–90 participants was sufficient [35].
Intervention
Patients reporting to the Pulmonary Function Testing Laboratory performed spirometry, and if all inclusion criteria and none of the exclusion criteria were met, they were randomly assigned to one of two therapeutic groups. Children in the first group received a standard salbutamol dose of 5 mg in 2.5 ml of nebulization solution (Steri-Neb Salamol, salbutamol sulphate solution, 5 mg/2.5 ml, IVAX Pharmaceuticals, UK) inhaled by continuous jet nebulizer (CON). In this group (CON group), the Porta-Neb compressor (MEDIC-AID, UK) with a PARI LC PLUS nebulizer head (PARI Medical Ltd, UK) was used (a typical device used for nebulization in our hospital) (Table 1) [36–38]. The residual volume (i.e. the volume of drug remaining in the nebulization chamber at the end of nebulization) of this nebulizer is 1 ml, therefore the total dose of salbutamol used per patient in this group was 7 mg (3.5 ml). Children in the second group received salbutamol from the breath-actuated jet nebulizer (BAN). In this group (BAN group), the MARIN MP3 compressor (Medbryt, Poland) with a RF6 PLUS nebulizer head (FLAEM NUOVA S.p.A., Italy) was used (Table 1). The salbutamol dose was halved in this group vs. the recommended dose for CON, i.e. to 2.5 mg (1.25 ml of drug solution). This was due to mathematical calculations (explanation in the discussion) and previous studies with BAN [39]. Also, the dose was increased in this group by the volume necessary to fill the residual volume of the nebulization chamber (0.9 ml). Therefore, a total of 4.3 mg of the drug was added into the nebulization chamber (2.15 ml of salbutamol solution). In both groups the drug was used without additional dilution. All subjects were previously instructed to maintain their natural breathing pattern until the medication was completely nebulized. Inhalation was performed using a mouthpiece. 15 min after the administration of salbutamol, the pulmonary function test was repeated.
Table 1
Children were observed for 1 h from the beginning of salbutamol nebulization. Their heart rate and blood pressure was assessed every 15 min. Elevated blood pressure was recognized based on percentile grid for the Polish population of school children and adolescents [40], and tachycardia based on available standards in children [41, 42]. In addition, symptoms reported by patients were recorded.
Spirometry
As mentioned above, a flow-volume loop was recorded before and 15 min after salbutamol nebulization using a KoKo PFT spirometer (nSpire Health, Inc., USA). Children performed spirometry three times and the curve with the best FEV1 and FVC values was selected for further statistical analysis. Changes in FEV1 and FEF25–75 after drug administration were calculated in relation to the baseline values in percentage (ΔFEV1, ΔFEF25–75) according to the formulas [43]:
Randomization
The randomization list was generated using Random Allocation Software. Participants drew a card with a unique number that assigned them to the appropriate group (CON or BAN).
Statistical analysis
The collected data were analysed using IBM SPSS Statistics 25. Outcomes of the two nebulization methods were compared using the Mann-Whitney U test. The χ2 test was used to check whether there is a significant relationship between the nominal variables. P-value of less than 0.05 was considered statistically significant.
Results
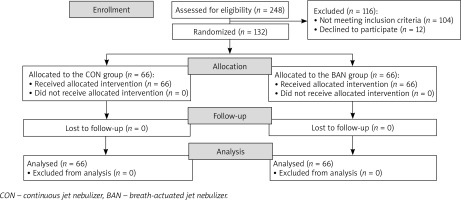
Participants were selected from among 248 children with bronchial obstruction admitted to the Pulmonology Outpatient Clinic at the University Children’s Hospital in Lublin. A total of 132 BDRTs were performed (Figure 1). The characteristics of the studied groups are summarized in Table 2.
Table 2
Demographic and clinical characteristics of the studied groups
| Parameter | CON group (n = 66) | BAN group (n = 66) | P-value |
|---|---|---|---|
| Age, mean ± SD [years] | 11.2 ±3.1 | 11.9 ±3.4 | > 0.05 |
| Sex, n, male/female | 44/22 | 46/20 | > 0.05 |
| IgE-dependent asthma, n (%) | 60 (90.9) | 63 (95.5) | > 0.05 |
| Baseline heart rate, mean ± SD [beats per minute] | 82.6 ±8.4 | 81.4 ±7.8 | > 0.05 |
| Baseline value of blood pressure [mm Hg] | 106/62 | 109/65 | > 0.05 |
| Baseline elevated blood pressure*, n (%) | 3 (4.6) | 2 (3.0) | > 0.05 |
| Asthma control medications, n (%): | |||
| Low dose ICS | 41 (62.1) | 39 (59.1) | > 0.05 |
| Medium dose ICS | 3 (4.6) | 2 (3.0) | > 0.05 |
| ICS + LABA | 22 (33.3) | 25 (37.9) | > 0.05 |
The mean baseline FEV1% was 70.5 ±8.9% in the CON group and 67.4 ±11.6% in the BAN group (p = 0.216). There was also no statistically significant difference between the compared groups in terms of baseline FEF25–75% (p = 0.067) (Table 3).
Table 3
Bronchodilator response to salbutamol in the CON and BAN groups
[i] FEV1% – forced expiratory volume in the first second as a percentage of the predicted value, FEF25–75% – forced expiratory flow at 25–75% of vital capacity as a percentage of the predicted value; ΔFEV1 or ΔFEF25–75 – changes in FEV1 or FEF25–75 after SABA administration in relation to the baseline values in percentage.
The ΔFEV1 was 16.9 ±9.7% in the BAN group and was statistically significantly higher than in the CON group (12.6 ±8.8%) (p = 0.026). The ΔFEF25–75 was 37.7 ±23.2% in the BAN group and 32.7 ±25.5% in the CON group (p = 0.061) (Table 3).
Adverse events were observed during the first hour after the beginning of nebulization in 9 patients (5 from the BAN group and 4 from the CON group). A total of 15 adverse events were recorded in the BAN group and 14 in the CON group. There were no statistically significant differences in the frequency of these symptoms between the compared groups of children (p = 0.68) (Table 4).
Table 4
Adverse effects observed during bronchodilator response tests with salbutamol in CON and BAN groups
Discussion
As mentioned earlier, jet nebulizers come in several varieties: conventional or continuous (CON), breath-assisted, breath-enhanced, breath-actuated (BAN) and breath-adapted [7, 17, 19, 44, 45]. Each group of jet nebulizers used with the same drug and at the same dose can produce significantly different pulmonary deposition and possibly a different clinical effect [18, 46, 47]. This was confirmed by Finlay et al., who showed that the lung deposition of salbutamol inhaled from 19 different nebulizers ranged from 3.1% to 23.4% (p < 0.01) of the nominal dose placed in the nebulizer [23]. In another study, Walz-Jung et al. showed significant differences between 9 different jet nebulizers during the nebulization of salbutamol. The drug delivery rate varied from 67 μg/min to 196 μg/min [48]. These differences result from significantly different technical parameters of individual components of nebulization devices, such as compressor, nebulizer head, and residual volume of the nebulization chamber. This, in turn, translates into the emitted dose and the characteristics of the aerosol cloud (mass median aerodynamic diameter – MMAD, fine particle fraction – FPF, geometric standard deviation – GSD) [49–51]. However, the final clinical effect of a nebulized drug also depends on the patient’s breathing pattern (tidal volume, respiratory rate, inspiration to expiration ratio) and the functional state of the patient’s airways [52, 53].
The relationship between MMAD and the bronchodilatory effect of salbutamol was demonstrated by Usmani et al. [16]. The researchers showed a greater bronchodilatory effect of this drug assessed by measuring FEV1 and FEF25–75 in adults with asthma during the inhalation of monodisperse aerosol with a MMAD 3.0 and 6.0 μm than that with a MMAD 1.5 μm. In our study, the MMAD of both aerosol clouds were slightly different (3.8 μm for CON, 2.8 μm for BAN). However, these values were similar to those which were effective according to the data from the study by Usmani et al. [16].
There are relatively few studies comparing the spirometric and clinical effects of salbutamol nebulization from various nebulizers in children with asthma [27, 29, 54, 55]. Much more reports concern the comparison of the efficacy of salbutamol nebulization with salbutamol administered via pMDI [56, 57]. This is due to the still low availability of BANs, the rapid development of this group of nebulizers only in the last 20 years, their large diversity, as well as the difficulty in assessing the actual lung deposition of inhaled drugs by in vivo studies in children.
BANs are usually characterized by greater lung deposition and a better clinical effect versus CONs. Nikander et al. in a series of their studies showed that in 5–15-year-old asthmatic children, the average mass of budesonide inhaled from the BAN ranged from 17.1% to 21.6% of the nominal dose [58–60]. In the case of nebulization of the same drug but from a CON, this value was two times lower (8.9% to 12.2%). Another study conducted by researchers from Taiwan provides similar observations [55]. They showed that the nebulization of the same dose of terbutaline using BAN is better than using CON in improving spirometric parameters in asthmatic children, which may be related to higher lung deposition of the drug in the first group. Wilkinson et al. recorded shorter lengths of stay in emergency departments in children with moderate to severe asthma exacerbation treated with BAN compared to CON (118 vs. 163 min, p = 0.0002), without differences with respect to admission rates, changes in asthma scores, albuterol side effects, or readmission rates [27]. On the other hand, the study by Parone et al. demonstrated no clinical difference between the bronchodilator nebulization from BAN and CON in adult patients with dyspnoea and wheezing [26]. In another study, albuterol delivered via CON resulted in a significantly greater improvement in FEV1 than albuterol delivered by a breath-enhanced nebulizer [29]. As shown above, the results obtained by the researchers are inconsistent.
The analysis of the nebulization process from the RF6 PLUS with MARIN MP3 device (BAN group) indicates that about 1.25 ml of the solution, i.e. about 2.5 mg of salbutamol (emitted dose) left the nebulization chamber. Bearing in mind that nebulization occurred only during the inspiratory phase, it should be assumed that about 2.5 mg of salbutamol reaches the patient’s respiratory tract (deposited dose) [36]. In the case of inhalation from the PARI LC PLUS with Porta-Neb device (CON group), the emitted dose was 2.5 ml of the solution, i.e. 5.0 mg of salbutamol. This inhaler provides continuous aerosol during inhalation, exhalation, and breath-holding, causing the release of aerosol to ambient air during exhalation (which is prolonged in a child with bronchial obstruction – up to 80% of the respiratory cycle) and anytime when the patient is not breathing. Due to the large loss of the drug caused by the characteristics of this inhaler, a maximum of 20% of the dose, i.e. about 1.0 mg of salbutamol (deposited dose) reached the respiratory tract [19, 58]. This may explain the weaker bronchodilatory effect in the CON vs. BAN group.
Our results are in line with those presented by Sabato et al., in which the dose of 2.5 mg of salbutamol from BAN was more clinically effective than 10 mg of this drug from CON, which was associated with greater deposition of the drug in the bronchi in the former group [54]. The evidence for the above is also provided by Nikander et al. [58]. Thus, in our study, the dose of salbutamol in the BAN vs. CON group was reduced twice. We showed a better bronchodilatory effect of salbutamol assessed by FEV1 in the group inhaling from BAN. The values of mid-expiratory flows assessed by FEF25–75 improved by over 30% in relation to the baseline values in both groups, more clearly in the BAN group, but these differences did not reach statistical significance. This can be explained by the characteristics of the aerosol clouds produced by both inhalers (Table 1), which indicate the possibility of a greater deposition of the drug in the larger bronchi [59, 60].
Various side effects associated with the use of nebulization forms of salbutamol have been reported in children with asthma, including hypokalaemia and lactic acidosis [61–63]. They were reported more often in children treated with SABA using the CON, than pMDI with VHC, and the main symptom was tachycardia [64, 65]. Our study showed similar rates of side effects in both groups despite the fact that twice the nominal salbutamol dose was used in the CON group than in the BAN group. Similar observations are provided by Sabato et al. and Wilkinson et al. [27, 54]. In turn, Lin et al. showed that administration of the same dose of terbutaline from two different types of jet nebulizers resulted in a higher heart rate in the BAN group vs. CON group, which the authors explain with greater lung deposition of the drug in the first group [55].
The work has some limitations. Firstly, in the CON group, the Porta-Neb compressor was used in combination with the PARI LC PLUS nebulizer head instead of the recommended Medic-Aid Sidestream (for rhDNase nebulization) or Medic-Aid Ventstream or Turret (for ICS nebulization) [37]. This was due to the technical and financial limitations of our hospital. However, it should be noted that the above recommendations regarding the combination of the Porta-Neb compressor with the appropriate nebulizer heads apply to the inhalation of rhDNase and ICS, while there are no such recommendations for SABA. Secondly, the dose of salbutamol for the BAN group was determined based on theoretical assumptions, because there were no literature data (algorithms) on how to convert SABA doses administered from CON to clinically equivalent doses administered from BAN. Thirdly, due to the different appearance and characteristics of the work of both nebulizers, it was impossible to blind the researcher and patients.