Introduction
The importance of vaccination in preventing epidemics and pandemics cannot be overstated. Vaccines safeguard individuals from infectious diseases, curbing their spread in the population, especially among high-risk groups. They also establish herd immunity, limiting the chances of viral mutations that could increase transmission or severity. Universal vaccination plays a pivotal role in halting these mutations, as fewer infections mean reduced mutation risk [1-3]. This significance is amplified for healthcare workers, who, by getting vaccinated, not only reduce their own infection risk but also protect patients and colleagues. This ensures uninterrupted medical care and less strain on healthcare systems. The COVID-19 pandemic exemplified this, prompting the vaccination of healthcare workers to curb infections, maintain care continuity, and mitigate the pandemic’s impact [4-7].
Safe and effective COVID-19 vaccines were introduced in the EU in late 2020, following rigorous safety protocols [8]. In Poland, the vaccination program began on December 27, 2020, starting with high-risk healthcare professionals. It was eventually extended to all medical staff and became mandatory on March 1, 2022 [9]. By April 18, 2023, Poland had administered over 4.8 million doses to healthcare workers out of a total of 57,966,780 vaccinations [10]. These vaccinations demonstrated their effectiveness, with vaccinated workers experiencing milder COVID-19 symptoms and faster recoveries compared to unvaccinated staff [11-13].
The aim of this study is to investigate the effectiveness of COVID-19 vaccinations among healthcare workers by assessing the levels of immunoglobulin G (IgG) anti- SARS-CoV-2 antibodies two years after their administration and analyzing the basic factors influencing these antibody levels.
Material and methods
Study group
For the purposes of this study, we analyzed data from serological tests conducted during the implementation of the project titled “Reducing the negative effects of COVID-19 through preventive and protective measures addressed to medical services” prepared by the Kujawsko-Pomorskie Voivodeship Self-Government. The study encompassed results obtained between September 9, 2022, and December 19, 2022, involving employees from hospitals, clinics, sanatoriums and sanitary and epidemiological stations.
Serological tests
The levels of anti-SARS-CoV-2 IgG were determined using ELISA, employing the EUROIMMUN I-2P automatic analyzer and the Anti-SARS-CoV-2 QuantiVac ELISA (IgG) kit from EUROIMMUN (Lübeck, Germany). The tests were conducted in accordance with the manufacturer’s recommendations and a detailed methodology described in our previous research [14]. The tests were performed in 96-well microplates coated with the SARS-CoV-2 S1 domain (including RBD, receptor-binding domain) recombinantly expressed in the human cell line HEK293 (ATCC). The research protocol and informed consent were approved by the Bioethics Committee of the Nicolaus Copernicus University in Torun at Ludwik Rydygier Collegium Medicum in Bydgoszcz (Approval No. KB173/20).
Statistical analysis
The statistical analysis was performed to assess the impact of COVID-19 occurrence, vaccination timing and type, and the number of vaccine doses on the concentration of anti-protein S IgG antibodies two years after the initiation of the COVID-19 vaccination program in Poland. PS Imago Pro software was utilized for the analysis. Initially, a descriptive analysis was conducted, followed by an analysis of variance (ANOVA), taking into account the factors such as the occurrence of COVID-19, vaccination timing and type, and number of doses. This was done to determine whether there were significant differences in IgG antibody concentration among the groups. In the case of significant differences, post-hoc analyses were performed using Tukey’s test to identify which groups exhibited significant differences from each other. Furthermore, a Pearson correlation analysis was conducted to explore the relationship between the number of doses and IgG antibody concentration, as well as the correlation between the time since vaccination and IgG antibody concentration. All analyses were carried out with a significance level of p < 0.05.
Results
Characteristics of the study group
The study encompassed data from 4,090 participants aged over 18, comprising 85.8% women and 14.2% men. The largest age group consisted of individuals aged 46 to 55 (32.3%) while the smallest group comprised individuals aged over 65 (6.0%). Regarding the occurrence of SARS-CoV-2 infection among participants, three categories were distinguished. Approximately 50.3% of individuals had confirmed COVID-19, with 4% testing positive twice. In total, 96.6% of respondents received at least one dose of a COVID-19 vaccine. The most frequently chosen vaccine was Pfizer/BioNTech (88.1%), while the least frequently chosen was Johnson & Johnson (2.9%). The majority (65.8%) received a booster dose, while only 3.4% of respondents were not vaccinated. Additionally, 21 subjects received only one dose of two-dose vaccines, rendering their vaccination incomplete (Table 1).
Table 1
Characteristics of the study group, taking into account the average concentration of anti-SARS-CoV-2 IgG against protein S
Vaccination preferences and dependencies
An analysis of the data revealed relationships between the choice of a specific vaccine and the occurrence of COVID-19, as well as between the number of doses and infection. Tables 2-5 summarize data on the number of participants who did not receive the COVID-19 vaccine and the number of individuals who received different types of vaccines (Pfizer/BioNTech, Moderna, AstraZeneca, Johnson & Johnson), as well as infection data. Unvaccinated individuals accounted for 3.4% of the group, with only 19 individuals testing negative for antibodies (9 had COVID-19, 10 reported no infection). The fewest individuals opted for vector vaccines (AstraZeneca, Johnson & Johnson). Among the 4,090 study participants, 85.1% chose the Pfizer/BioNTech vaccine. The preference for this vaccine may be attributed to its recognized effectiveness and safety as endorsed by health organizations. The lack of confidence in AstraZeneca may be linked to concerns regarding its effectiveness and its potential impact on blood clot formation.
Table 2
Comparison of data on infections and vaccinations among study participants
Table 3
Distribution of antibody concentration depending on the vaccine type
Table 4
Distribution of antibody concentration depending on the number of doses administered
| Doses | Percentage of count | Median | SD | IgG concentration (BAU/ml) |
|---|---|---|---|---|
| 0 | 3.45 | 391.4 | 1382.11 |  |
| 1 | 3.11 | 839.2 | 1160.24 |  |
| 2 | 19.12 | 1191.8 | 1412.69 |  |
| 3 | 65.82 | 1711.9 | 1793.61 |  |
| 4 | 8.51 | 4287.8 | 2232.59 |  |
| 0 2000 4000 6000 8000 |
Table 5
Changes in antibody levels depending on time since last vaccine dose
Table 6
Correlation coefficients (r values) between the number of vaccine doses received, IgG concentration (BAU/ml), the quarter of the last vaccination, and the quarter of the last infection
Approximately 5% of all participants chose the Pfizer/BioNTech vaccine as a booster after being fully vaccinated with other vaccines. The largest group (65.8%) received three doses of the vaccine (Tables 1, 2, 4). About half of the study participants (Table 2) contracted COVID-19. In the unvaccinated group, 61% tested positive at least once, with 8.5% testing positive twice. In the vaccinated group, only 46.8% tested positive test at least once, and a mere 3.8% tested positive twice. Of these, 36.2% tested positive after completing the full vaccination course.
Most post-vaccination infections occurred between January and April 2022. The percentage of vaccinated individuals with confirmed infection was higher among those who received one dose of the vaccine compared to employees who received two doses. However, it should be noted that that percentage of vaccinated individuals who tested positive for the virus was consistently low for all vaccines (between 1.1% and 3.5%), underscoring vaccines’ effectiveness in preventing symptomatic SARS-CoV-2 infection.
Analysis of changes in antibody levels
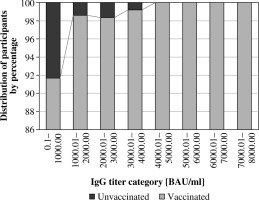
The study analyzed results of tests measuring antibodies against the SARS-CoV-2 virus in participants’ blood serum (Tables 3-5). The levels of IgG anti-S in the study population varied, with only 0.46% testing negative for the presence of antibodies. Results were categorized based on the titer of measured antibodies (Fig. 1). The largest percentage of individuals (15.6%) had an IgG result between 1000 and 2000 BAU/ml, with 14.3% of participants in the first category having a confirmed infection. For other IgG result ranges, the percentage of individuals with COVID-19 ranged up to 4.4%. However, it is worth noting that in some categories (e.g., from 5000 to 7000 BAU/ml), the number of participants tested was low, which may impact the results’ reliability. The distribution of individuals with and without virus exposure remained consistent across categories.
Fig. 1
Frequency of IgG anti-SARS-CoV-2 concentration categories among vaccinated and unvaccinated participants, comparing the percentage of individuals in divisions based on IgG levels every 1000 BAU/ml
An analysis of changes in anti-SARS-CoV-2 IgG concentrations depending on the time since the disease (Fig. 2), vaccination and number of doses (Table 5, Fig. 2) was performed. The analysis considered quarterly time intervals. As previous observations have indicated that antibody dynamics become relatively small 6-8 months after exposure to the SARS-CoV-2 antigen, quarterly analysis provides a comprehensive overview of results over an extended period, and facilitates comparison between quarters.
Fig. 2
Dynamics of IgG antibody levels after COVID-19 vaccination in relation to doses and prior COVID-19 infection. The data have been divided into two groups: individuals who did not experience COVID-19 prior to vaccination (A) and those who had previously contracted COVID-19 (B). The analysis also takes into account the impact of the number of vaccine doses administered and divides time into quarters, enabling a comprehensive understanding of the dynamics of antibody level changes over time

Test results revealed significant differences in the average anti-SARS-CoV-2 IgG values among quarters (Fig. 2). The highest average concentration of anti-SARS-CoV-2 IgG was observed in the first quarter after the disease, measuring 3647.11 BAU/ml (n = 122, SD = 2321.92). Subsequent quarters saw gradual decreases: 2601.49 BAU/ml (n = 71, SD = 1789.68) for the second quarter, 2394.60 BAU/ml (n = 505, SD = 1968.33) for the third quarter, and 2103.77 BAU/ml (n = 322, SD = 1595.59) for the fourth quarter. The fifth quarter recorded a slight increase to 2407.69 BAU/ml (n = 75, SD = 2189.66), but a significant decrease followed in the sixth quarter to 1565.22 BAU/ml (n = 24, SD = 1299.17). In the seventh and eighth quarters average values stabilized at 1856.53 BAU/ml (n = 174, SD = 1713.01) and 1865.50 BAU/ml (n = 658, SD = 1635.95), respectively. The ninth quarter saw a slight increase to 1917.33 BAU/ml (n = 232, SD = 1630.61), but the tenth quarter marked a significant decrease to 1376.47 BAU/ml (n = 11, SD = 602.24). Statistical analysis confirmed significant differences in average anti-SARS-CoV-2 IgG values between consecutive quarters from disease onset (p-value < 0.001).
Subsequently, the analysis examined changes in the IgG antibody level against SARS-CoV-2 protein S based on the time since the last vaccine dose (Fig. 2, Table 5). Eight quarters were analyzed, each representing the time since the last vaccine dose. The first quarter exhibited an average IgG value of 4378.70 BAU/ml (n = 359, SD = 2263.21), which decreased to 2900.47 BAU/ml (n = 107, SD = 2195.76) in the second quarter. The third quarter recorded further reduction to 2003.37 BAU/ml (n = 564, SD = 1629.50), followed by 2155.77 BAU/ml (n = 1792, SD = 1761.41) in the fourth quarter. The fifth quarter demonstrated a similar IgG value to the fourth (2150.34 BAU/ml, n = 637, SD = 1823.10). The sixth quarter exhibited a significant decrease to 1287.11 BAU/ml (n = 215, SD = 1238.53), followed by 1821.48 BAU/ml (n = 238, SD = 1412.95) in the seventh quarter. The eighth quarter saw IgG at 2,017.37 BAU/ml (n = 37, SD = 1,642.94), with the mean total IgG measuring 2,286.65 BAU/ml (n = 3,949, SD = 1,908.31). Statistical analysis confirmed significant differences in IgG antibody levels between consecutive quarters (p-value < 0.001). Examining the results, it is apparent that IgG anti- body levels were highest in the first and second quarters, followed by gradual decreases. Similar IgG levels were observed in the fourth and fifth quarters, followed by a significant decrease in the sixth quarter. However, IgG levels remained relatively high in individuals vaccinated in subsequent quarters, indicating a sustained immune response.
The analysis of the IgG antibody levels against SARS-CoV-2 spike protein, depending on the number of vaccine doses administered, revealed statistically significant differences in the mean values (Table 4). As expected, the greater the number of doses received, the higher was the average concentration of measured antibody levels. Individuals who received four vaccine doses exhibited an average antibody level more than twice as high as those who took three doses, and four times higher when compared to unvaccinated individuals who developed antibodies following natural infection. Those who received three doses, on average, had antibody levels two times higher than individuals who received only one dose or none (Table 4).
The influence of variables on the level of antibodies
The multivariate scatterplot analysis provided valuable insight into the relationship between time since COVID-19 onset (Fig. 2A), time since the last vaccination (Fig. 2B), and antibody concentration. It revealed that longer periods since infection correlated with antibody concentration. A similar trend was observed for time since the last vaccination, although the effect was less pronounced. As expected, a higher number of vaccine doses correlated with higher antibody titers. Notably, there were no significant differences in antibody titers between individuals who had contracted the disease and those had not. This may be attributed to asymptomatic infections among fully vaccinated individuals and a high level of immunization in vaccinated individuals, where infection did not significantly increase antibody levels.
Correlation analysis expressed by the Pearson correlation coefficient revealed moderate correlations for all variables. The correlation between IgG concentration and the number of vaccine doses was 0.339** and was significant at p < 0.001. The correlation between vaccination quarter and IgG concentration was –0.282** and was significant at p < 0.001. The correlation between the quarter after infection and IgG concentration was –0.171** and was significant at p < 0.001.
Discussion
Healthcare workers were prioritized for COVID-19 vaccination during the pandemic. Research has shown that the vaccination attitude of healthcare professionals varies based on several factors. Factors such as gender, age, education level, perceived risk, direct patient care roles, and prior influenza vaccination history influenced vaccination rates positively [15]. Conversely, individuals with lower incomes, less control over their work schedules, and lower confidence in vaccine efficacy were less likely to get vaccinated [16]. Medical facilities in Poland achieved high vaccination rates swiftly due to the organized and aware nature of healthcare workers, ease of vaccine access on site, centralized management, and their influential role in the community. The rapid development of vaccines, including mRNA-based ones, has raised questions about their effectiveness and their impact on the immune response. Research teams worldwide, including ours, have undertaken studies to assess the immunological status of vaccinated individuals, with a primary focus on healthcare professionals [17-20]. Antibody level determination, mainly IgM, IgG, and IgA classes, plays a pivotal role in assessing population or herd immunity. It aids in identifying prior virus exposure or IgG production due to vaccination. High antibody titers across a population suggest acquired immunity and protection against further disease transmission, which is particularly valuable for SARS-CoV-2, given its potential for asymptomatic or mild cases.
Our study included 4,090 healthcare workers aged 18 and older, with nearly half of them reporting prior SARS-CoV-2 infection. Most participants received at least one dose of COVID-19 vaccine, and Pfizer/BioNTech was the most frequently administered vaccine. This preference for Pfizer/BioNTech likely stems from its recognized effectiveness and safety, along with its prioritization for healthcare workers as the first available option. Only 3.4% of respondents remained unvaccinated, and merely 19 of those who had not been vaccinated tested negative for antibodies. Our research indicates that individuals vaccinated against COVID-19 maintain high levels of IgG antibodies against the SARS-CoV-2 S protein. These levels remain robust for up to 6-8 months after full vaccination and persist for at least 2 years following booster doses. During this timeframe, breakthrough infections may occur but generally exhibit milder symptoms. This sustained immune response underscores the vaccine’s protective efficacy. Additionally, a higher number of vaccine doses corresponded to elevated antibody levels. Furthermore, as the time since COVID-19 onset or the last vaccination increased, antibody concentrations tended to decrease, albeit with a less pronounced effect. Approximately 65.4% of participants received three vaccine doses. Notably, the time since the last vaccination and the number of doses administered exerted the most substantial influence on IgG antibody levels. After two years of universal vaccination, the role of prior illness in the early vaccine response declined [21, 22].
The rising global occurrence of SARS-CoV-2 reinfections is linked to the emergence of more challenging-to-combat mutations, raising the risk of recurrent infections. Protection against these variants hinges on both innate and adaptive immune responses. However, factors such as immunity waning within 6-12 months after infection may facilitate the development of more infectious variants, elevating re-infection risks. Addressing these challenges, including understanding immune protection, monitoring new SARS-CoV-2 variants (VOCs), and maintaining adaptive immunity, necessitates the development of effective therapeutic and preventive strategies [23, 24].
The time factor, with several months having passed since the onset of COVID-19, and the presence of asymptomatic or mildly symptomatic infections among vaccinated individuals (latent infections) might explain the observed outcomes. Accurately assessing the percentage of infections after vaccination is challenging, especially as common tests are discontinued. In our study, around half of the participants contracted COVID-19, of which 36.2% tested positive after completing the full vaccination regimen. Notably, those who received only one vaccine dose had a higher proportion of confirmed infections compared to those who received both doses, underscoring the importance of completing the full vaccine schedule. Additionally, individuals who remained unvaccinated exhibited lower antibody concentrations compared to fully vaccinated counterparts, further highlighting the immunological benefits of vaccination programs.
Our study revealed a high vaccination rate among participants (96.6%). Remarkably, nearly 3% of unvaccinated individuals demonstrated the presence of post-COVID antibodies resulting from natural infection. Considering the number of individuals with antibodies due to SARS-CoV-2 exposure, it appears that medical unit employees have approached herd immunity. Nevertheless, the relatively high percentage of vaccinated workers who contracted COVID-19 implies a shortfall in individual and population immunity. Our findings suggest that vaccination conferred some benefit, as the proportion of positive COVID-19 cases among vaccinated individuals (once or twice) was significantly lower than in the unvaccinated group. However, it is crucial to note that vaccination does not guarantee complete protection against infection but rather reduces the risk of severe COVID-19. The effectiveness of vaccinations may vary based on factors such as health status, age, virus mutations, or the time between vaccine administration and the spread of mutated viruses (which was not analyzed in this study). Therefore, further research is essential to comprehend the factors influencing vaccine effectiveness and to determine precautions for minimizing infection and virus spread.
Definitive claims about herd immunity within medical environments cannot be made based on the provided data alone. A more comprehensive data analysis and context evaluation are necessary. Herd immunity hinges on several factors, including the number of infected medical workers in a unit, adherence to safety protocols by staff, and vaccination rates among medical personnel. Based on our research, medical units with a high vaccination rate and individuals with previous natural COVID-19 infection appear to exhibit herd immunity to the SARS-CoV-2 virus. After vaccination or recovering from COVID-19, most individuals develop antibodies that reduce the risk of infection and disease transmission within the medical environment. However, it is important to acknowledge that emerging virus mutations (such as delta and omicron) have led to breakthrough infections, potentially compromising the efficacy of existing vaccines. There remains a risk of immunocompromised individuals, including patients, being infected within medical units, contributing to further virus transmission. Therefore, continuous monitoring and adaptability of COVID-19 strategies are essential to ensure the protection of medical staff and patients.
To sustain effective protection against COVID-19, booster doses are crucial, given the gradual decline in antibody levels over time and its impact on vaccine efficacy. In our medical community study, over 60% of individuals received their third booster dose, and an additional 8.5% opted for an extra booster. As new SARS-CoV-2 mutations emerge, vaccine effectiveness evolves. Notably, Gilboa et al. affirmed a significant rise in antibody and T-cell levels following booster doses, indicating longer-lasting immunity to COVID-19. However, it is important to acknowledge that booster vaccines, while effective in preventing severe omicron cases, may not entirely prevent infection or mild illness [25]. In the case of mRNA vaccines, updates can be relatively swift and straightforward, enabling the production of vaccines with broader immunization. Booster doses serve the dual purpose of maintaining and augmenting protection among those previously vaccinated, which is instrumental in curbing virus transmission and safeguarding public health.
As of 2022, healthcare workers remain at risk of COVID-19 infection despite vaccination program implementations in many countries. The evolving pandemic and virus mutations have led to reports of breakthrough infections among vaccinated individuals, including healthcare workers. Research by the Centers for Disease Control and Prevention (CDC) revealed approximately 12.4 million COVID-19 cases in the United States between May 1, 2021, and January 31, 2022, with 31% occurring in vaccinated individuals. Nonetheless, vaccinated individuals are less likely to experience severe complications or fatalities from COVID-19, as per CDC findings. Notably, studies by Yigit et al. [26] and Gilboa et al. [25] underscore the substantial increase in antibody levels after booster doses, significantly enhancing protection against new SARS-CoV-2 variants. These findings emphasize that while vaccines may not achieve 100% effectiveness against breakthrough infections, they remain a valuable tool in preventing severe illness and death [27].
Cohort studies have indicated that subsequent doses of vaccines result in a 2-3-fold increase in antibody levels and effectively prevent COVID-19 for at least 6 months. Booster vaccine doses have emerged as a potent tool in combatting and mitigating the impact of breakthrough infections. Consequently, breakthrough infections represent a smaller proportion of cases compared to re-infections among unvaccinated individuals [28-31]. Yang et al. [32] conducted an analysis of breakthrough infections among vaccinated healthcare workers, assessing the humoral response in individuals experiencing such infections. The study encompassed 251 healthcare workers, 18 of whom had breakthrough COVID-19 infections despite vaccination. The study’s findings revealed that individuals with breakthrough infections displayed lower antibody levels against the SARS-CoV-2 virus than those without such infections. Nevertheless, the antibody levels in individuals with breakthrough infections remained sufficiently high to provide protection against COVID-19.
These findings carry significant clinical implications for vaccine strategies and the monitoring of immune responses in COVID-19 patients. Identifying the optimal timing between infection and vaccination can enhance vaccine effectiveness and reduce the risk of re-infection. Consistent monitoring of antibody levels in COVID-19 patients enables the identification of individuals in need of additional vaccine doses to bolster their immunity. The vaccination of healthcare workers has played a pivotal role in ameliorating the COVID-19 situation by reducing infection rates among these professionals, thereby mitigating the risk of virus transmission to patients and the broader community. However, further research is needed to delve deeper into factors influencing vaccine effectiveness and infection risks, especially in the context of emerging virus mutations.
Our understanding of the immune response to COVID-19 infection and vaccination has evolved significantly since the pandemic’s onset in March 2020. While post-infection immunity offers some protection, it wanes over time, especially against new virus variants, leading to reinfections. Vaccination has been instrumental in reducing infections and deaths, but the emergence of new variants and declining immunity necessitate additional vaccine doses. These booster shots enhance protection for both vaccinated and previously infected individuals. Our study underscores the importance of monitoring antibody levels in vaccinated individuals and the potential need for additional vaccine doses to maintain adequate protection. Additionally, our findings confirm the effectiveness of vaccination in safeguarding healthcare workers from COVID-19 and highlight the advantages of mRNA vaccines.
Overall, our research offers valuable insights into the long-term effectiveness of COVID-19 vaccines and emphasizes the critical role of vaccination in protecting healthcare workers and the broader population against the virus.