Dear Editor,
Cannulation with central venous access catheters in hospitalized patients is a routine procedure, especially in the critical care area. However, the procedure that is not exempt from complications, some of them with serious repercussions for the life of the patient, and its success depends largely on the experience of the professional [1–3].
The incidence of complications related to the catheterization of a central vein vary between 10% and 20%. Cardiac tamponade is one example of such serious sequela, albeit very rare. Its incidence ranges between 0.14% and 0.30% with a high mortality rate, and from 37.5% to 100% if there is ventricle perforation [4, 5].
We present the case of a patient with cardiac tamponade, secondary to a first central venous access by ultrasound-guided Seldinger technique for the administration of parenteral nutrition.
An 84-year-old Spanish woman with a medical history of arterial hyper-tension, dyslipidaemia, and mild cognitive impairment with suspected Alzheimer’s disease was admitted to the hospital ward for a month due to severe acute cholecysto-pancreatitis, awaiting endoscopic retrograde cholangiopancreatography and qualified for parenteral nutrition for which a central venous catheter (CVC) was needed.
After laboratory test (with no apparent contraindications detected), obtaining consent, and aseptic preparation of the skin, a single, successful, ultrasound-guided puncture of the right internal jugular vein was performed under local anaesthesia with the patient in the Trendelenburg position. The Seldinger technique was used for cannulation of the vessel (triple-lumen 30 cm, 7 Fr, J-tip). During the procedure, the patient remained agitated due to poor tole-rance of the position and associated discomfort, as well as prior disorientation. After insertion of the catheter, parenteral nutrition was started following radiographic verification. Two minutes later, while the central line was being sutured to the skin, a decreased level of consciousness, agonal breathing, and bilious vomiting followed by respiratory arrest were observed. Urgent manoeuvres were performed to control the airway, escalating to emergency orotracheal intubation, with maintenance of arterial pulse and auscultation with bilateral preserved vesicular murmur.
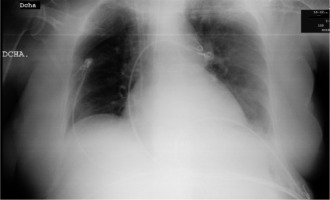
Upon admission to the ICU the patient remained mechanically ventilated. The presence of pneumothorax was ruled out after performing serial urgent chest X-rays (Figures 1 and 2); in the first of these images the left heart border is very sharp and pronounced. The quality of the image did not allow differentiation between haemopericardium and combined haemo-pneumopericardium.
It was also possible to observe haemodynamic instability with severe hypotension refractory to intensive fluid therapy and norepinephrine up to 2.9 mg kg -1 min-1 (maximum systolic blood pressure 75 mmHg). Intravenous hydrocortisone was prescribed for refractory shock of unknown origin at that time. Twelve-lead electrocardiogram showed sinus tachycardia without findings of interest (Figure 3). In ABG, mixed acidosis with elevated lactates was seen, for which intensive serum therapy was maintained and 8.4 g of bicarbonate was administered due to persistence of alterations in the acid-base balance. Of note were also anuria without response to diu-retics, absence of anaemia, no obvious bleeding, and normal coagulation. An emergency transthoracic echocardiography was performed due to refractoriness of the shock, showing dynamic obstruction to the flow of the left ventricular outflow tract by mitral leaflets and pericardial haematoma at the level of the right ventricle in the subxiphoid window (Figure 4) consistent with diagnosis of cardiac tamponade and cardiogenic shock. Given these findings, urgent pericar-diocentesis by ultrasound-guided subxiphoid approach was considered, being dismissed due to the clinical situation and not having a cardiac surgery service in our centre (reference hospital 2 hours 30 minutes by ambulance).
Poor response to treatment was discussed with the relatives, agreeing on the adequacy of life support measures. The patient died 4 hours after admission to the ICU.
Infections, thrombosis, and local trauma constitute the most important complications associated with the use of these catheters. Complications directly attributable to catheter placement occur in 3–12% of cases. The rate of complications due to the implantation and maintenance of the catheter is 12.5–20% of cases (14.7 complications per 1000 catheter-days).
The most frequent complications when cannulating the internal jugular vein are due to local trauma (potentially any structure in the vicinity of the vein may be injured), haematoma due to puncture of the internal carotid artery, pneumothorax due to pleural puncture, haemothorax, primary or secondary malposition, and cardiac arrhythmias [6]. Less common complications include air embolism, Horner’s syndrome, damage to the brachial plexus, or perforation of the cardiac chambers [7]. Anecdo-tally, extraordinary sequalae such as paraplegia due to infusion of parenteral nutrition into the spinal canal, formation of a pseudoaneurysm due to puncture of the vertebral artery, and cardiac tamponade secondary to thrombosis of the coronary sinus have been reported [8].
Complications that result directly from insertion are more closely related to the clinician’s experience than to the route or catheter chosen. Inexperienced clinicians have rates of cannulation failure or complications that are twice that of experienced clinicians [9]. In turn, the rate of serious complications increases dramatically when 3 or more independent skin punctures need to be performed [10].
It is difficult to determine the real incidence of cardiac tamponade, although it seems to be more frequent in children than in adults, because in the former the wall of the atrium and right ventricle are very thin and therefore more susceptible to trauma [11].
Until 1986, according to Karnauchow, 49 cases of cardiac tamponade due to central venous catheterization were described in the English literature [12]. In the period from January 1996 to August 1997 Collier reported 21 new cases, which indicates its increasing incidence [13].
There are several possible mechanisms involved in the occurrence of cardiac tamponade: direct trauma at the time of insertion, migration of the catheter, and mechanical or chemical erosion. At the time of insertion, a tear in the wall of the superior vena cava may occur at the junction with the right atrium (intrapericardially) or perforation of the right heart chambers – both the atrium and the ventricle. In the case of our patient, and according to the echocardiographic findings, the latter option seemed the more likely cause [14–16]. Movements, especially in flexion of the neck and head, and movement of the heart and diaphragm in the cephalad direction (e.g. during respiration) can cause migration of the catheter, potentially exacerbated by the great anatomical variations in the length of the superior vena cava. Additionally, the hypertonicity of parenteral nutrition solutions has been suggested as a factor responsible for the erosion of the vein wall, in turn causing conditions such as hydrothorax or tamponade, because if the tip of the catheter rests close to the wall, there is no dilution of nutrition with the blood flow [17]. When the tip of the catheter protrudes into the wall, mechanical and chemical irritation act synergistically. The presence of hypertonic solutions in the pericardium causes a rapid accumulation of fluid in the said space, due to an osmotic gradient [18, 19]. In the case described, although the placement of the catheter was primarily for administration of parenteral nutrition, it was never started due to the clinical circumstances and the lack of initial imaging tests.
Stiffer catheters, made from polyethylene or similar materials, were suspected of contributing to this pathology, but with the introduction of softer catheters made of polyurethane or silicone polymers (our catheters are made of the latter material) this complication has not disappeared [20].
In the internal jugular approach, right-sided cannulation is recommend-ed due to the higher success rate and fewer complications. Likewise, we should recommend catheters that are as short as possible, leaving their tip in the superior vena cava and above the right atrium, because tamponade rarely occurs at this level compared to placing it in the atrium or ventricle itself [21]. With our patient we used a 30-cm catheter, as shorter catheters (20 cm for example) were not available in our service.
Although a normal chest X-ray does not rule out late complications, it should be obtained to confirm the position of the catheter at the time of insertion (Figures 1 and 2), which should also be reviewed whenever an X-ray is performed for another reason. In some cases, aberrant locations such as in the azygos vein, hemiazygos vein, or internal mammary vein can only be appreciated by a lateral radiograph, because on the AP chest radiograph they seem to present a correct location. Moreover, normal cardiac silhouette does not rule out the presence of pericardial effusion [22].
There are several definitions for the correct placement of the catheter by radiological control, each with its downsides. Greenall et al. [23] suggest that the tip of the catheter should be no more than 2 cm below a straight line drawn between the lower borders of the medial ends of both clavicles on a standing postero-anterior radiograph. However, in the interpretation of the anteroposterior plates in the setting of the ICU, in which the patient remains supine, with the ray beam closest to the structures located anteriorly and peripherally, anterior structures are increased by 20% (parallax effect). Attempts have been made to solve this by proposing the junction between the right main bronchus and the azygos vein as a landmark (although this is usually not seen, it is known that its junction with the superior vena cava rests at an angle formed between the right main bronchus and the trachea), or at least avoid any location of the catheter tip that can be seen within the cardiac silhouette on a posteroanterior chest X-ray. The problem is that the border between the superior vena cava and the right atrium cannot be defined on conventional radiography [24].
Another published study, based on the results of autopsies, states that the carina is the best anatomical landmark with which to locate the tip of the catheter and avoid complications such as tamponade (its position does not change in respiratory pathology, it is practically in the same plane as the superior vena cava avoiding any parallax effect, and is usually visible even on a poor quality radiograph) [25, 26].
Although the withdrawal of blood through the catheter does not exclude cardiac tamponade, the inability to draw blood through it as well as obtaining milky material by thoracocentesis in patients with fat emulsions (parenteral nutrition) when chylothorax is not suspected, a pleural fluid/serum glucose gradient > 1, or the presence of erratic central venous pressures, are indications suggestive of perforation.
The delay in the appearance of signs or symptoms after insertion can confuse the clinician due to the performance of another series of diagnostic or therapeutic procedures during this interval. One-third of tamponades secondary to central catheter cannulation occur in the first 24 hours (suggesting that catheter tip penetration occurred at the time of insertion and not due to migration or erosion) and most in the first week after insertion. Beck’s triad (hypotension, tachycardia, high central venous pressure) may be absent, and in more than 29% of cases, death from cardiovascular collapse may occur suddenly, with only subtle preceding signs.
Replacement of the catheter over the guidewire is a controversial practice. It seems to involve an increase in the frequency of bacterial colonization of the catheter and of bacteraemia if we compare it with a new venipuncture. Mechanical complications with over-the-wire catheter exchange, including cardiac tamponade, are extremely rare [27].
It seems unbeneficial to perform a control X-ray after the change of access through the guidewire if it has not been complicated, is performed by experienced personnel, in monitored patients, and with stable vitals. If pericardial tamponade appears, it is suspected clinically rather than by radiological findings [28].
On the other hand, in relation to the length of the guidewire inserted in the vein (in this case, it was inserted up to 15 cm), in the case of first insertion and replacements in the subclavian vein and in the jugular veins, it seems that between 16 and 18 cm should be considered the upper limit. In turn, a better guide/catheter size correlation with the anatomy of each patient is necessary [29].
In our example, the special characteristics of the patient (advanced age, previous medical history, and poor evolution of the underlying disease) as well as the characteristics of our centre (lack of areas enabled to perform these invasive procedures with monitored patients, as well as a car-diac surgery service) made it impossible to treat the complication in any other way. The possibility of performing an echocardiography allowed us to make an early diagnosis, but performing a pericardiocentesis was ruled out due to the circumstances described. Although cardiac tamponade secondary to the insertion of the guidewire could be suspected (possible tearing of the wall of the superior vena cava at the junction with the right atrium or perforation of the right heart chambers), it could not be definitively confirmed because no autopsy was performed.