High flow nasal cannula (HFNC) is an alternative device for oxygenation, which improves gas exchange and reduces the work of breathing [1]. In patients with acute respiratory failure of various origins, HFNC shows better comfort and oxygena-tion than standard oxygen therapy delivered through a face mask [2].
Postextubation respiratory failure is common and causes increased morbidity and mortality. The reintubation rate is very variable but may reach 20% or more [3]. It has been related to respiratory mechanics, airway patency, and protection. In fact, adequate cough strength, minimal secretions, and alertness are necessary for successful extubation [4]. Moreover, a randomised controlled trial has shown a significant reduction in the reintubation rate for patients treated with HFNC as compared with standard oxygen [5]. In another large-scale trial, HFNC was equivalent to NIV in patients at high risk of extubation failure [6]. Even if the ideal treatment for prevention of reintubation has yet to be determined for high-risk patients, HFNC may be considered as a reference therapy during the postextubation period [7].
HFNC has been widely employed during the COVID-19 pandemic [8, 9]. However, no data have been published about post-ventilation management. Furthermore, weaning failure prediction tools, such as ROX index, have not been validated yet for COVID-19.
The purpose of this paper is to report a single-centre experience on the effectiveness and safety of HFNC in the weaning of COVID-19 patients.
METHODS
This was a cross-sectional, observational case series. The study was approved by the local Ethics Committee of University of Campania “Luigi Vanvitelli” and A.O.R.N. Ospedali dei Colli in accordance with the 1976 Declaration of Helsinki and its later amendments. Written informed consent was obtained from all subjects. We retrospectively analy-sed patient records from the Subintensive Care Unit of Cotugno Hospital, Naples, Italy. Nine patients were admitted for severe acute respiratory failure and interstitial pneumonia. SARS-CoV-2 was confirmed by real-time polymerase chain reaction (RT-PCR) on nasopharyngeal swab. All patients showed a typical progressive stage at chest imaging. Pharmacological treatment was administered according to local guidelines as a rescue measure because no specific anti-SARS-CoV-2 drugs were available (Table 1).
TABLE 1
Patients outcomes
All patients underwent mechanical ventilation (5 Helmet CPAP, 4 invasive mechanical ventilation). A substantial duration of ventilation (14 ± 3.5 days) was needed until improvement of gas exchange. Weaning was initiated following a stable period of ventilation. Nevertheless, SpO2 and pO2 when weaning directly to standard oxygen therapy were unsa-tisfactory with dyspnoea and signs of respiratory fatigue. Considering age, comorbidities, spontaneous breathing trial failure, and prolonged mechanical ventilation, we assumed that our patients were at high-risk of intubation.
We implemented a switch to HFNC set at 34–37°C and a flow ranging from 50–60 L min-1. Temperature and flow were set considering the patient’s comfort [10, 11]. Delivered FiO2 was set to achieve a target of SpO2 ≥ 95% (93% in the case of pre-existing COPD). Blood gases were performed daily, as well as assessment of dyspnoea, respiratory rate, heart rate, blood pressure, oxygen saturation, and patient comfort. The ROX index is the ratio of oxygen saturation/FiO2 to respiratory rate [12]. It is currently used to evaluate HFNC efficacy on avoiding ventilation in patients with acute respiratory failure and pneumonia. A ROX index less than 2.85 at two hours is a predictor of HFNC failure. In our protocol, ROX index was calculated at two hours to assess HFNC failure promptly.
All patients were persistently positive for SARS-CoV-2 at the time of HFNC initiation, as assessed by RT-PCR. During the protocol the patients stayed in single isolation rooms.
Categorical data were expressed as number and percentage, whilst continuous variables as mean and standard deviation (SD). Differences before and after HFNC treatment were tested, according to the normal distribution, by the parametric paired Student’s t-test. A P-value < 0.05 was considered statistically significant.
RESULTS
Nine patients (4 females; age 63 ± 13.27 years; BMI 27.2 ± 4.27 kg m-2) showed ARDS and needed ventilation. Frequent comorbidities were as follows: systemic blood hypertension (5/9), type 2 diabetes (2/9), COPD (2/9), coronary artery disease (1/9), atrial fibrillation (1/9), hypertrophic cardiomyopathy (1/9), as reported in Table 1. All patient underwent high-resolution chest computed tomography at baseline that showed a progressive stage of disease, with diffuse bilateral subpleural ground-glass opacities. Other common findings were consolidations and traction bronchiolectasis. All patients experienced a radiological improvement during ICU stay. Baseline PaO2/FiO2 was 109 ± 45 mm Hg. After a long course of ventilation all patient improved until a stable mean ventilation PaO2/FiO2 of 336 ± 72 mm Hg.
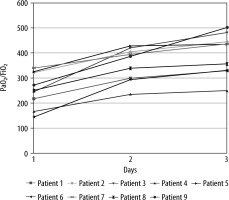
Right after initiation of HFNC (2 hours), PaO2/FiO2 was 254 ± 69.3 mm Hg (Figure 1). No signs of respiratory distress were observed; in fact, the respiratory rate was stable and ranged between 18 and 22 on HFNC (vs. 20–24 on ventilation). Mean ROX index at two hours was 11.17 (range: 7.38–14.4). It was consistent with low risk of HFNC failure. No difference was observed on lactate when patients switched to HFNC (1.72 ± 0.77 vs. 1.27 ± 0.46 mmol L-1; P = NS).
After 48 hours of HFNC oxygen therapy (day 3), PaO2/FiO2 significantly increased compared to day 1, with a mean of 396 ± 83.5 mm Hg (± 142 mm Hg; P < 0.0001). All patients recovered from respiratory failure at rest (PaO2 > 60 mm Hg in room air) after 7 ± 4.1 days. Patients outcomes are reported in Table 1.
During the HFNC period it was possible to perform a relevant rehabilitation plan. Initially all patients received respiratory physiotherapy and mobilisation, followed by active physiotherapy.
DISCUSSION
Soon after initiation of HFNC (2 hours) the mean PaO2/FiO2 was lower than previous ventilation values. Having a constant FiO2, this was probably caused by the lower PEEP delivered in HFNC [13]. The maximum PEEP during HFNC is estimated at about 5 cm H2O, while the mean PEEP applied during ventilation was 10 cm H2O [14]. All patients were stable and showed no signs of distress or intolerance. In fact, respiratory rate and lactate were stable when patients switched to HFNC. ROX index was consistent with low risk of HFNC failure, suggesting its reliability could be extended to COVID-19.
As per our experience, HFNC was deemed efficient after 48 hours of therapy. Efficacy was determined as a combination of a continuous upward trend of PaO2/FiO2 (Figure 1) with a good tolerance. Indeed, on day 3 the PaO2/FiO2 increased to a mean of 396 mm Hg. Thereby, it exceeded the average level during ventilation.
After this stabilisation step, we progressively decreased FiO2 day by day according to blood gas values [15]. All patients recovered from respiratory failure at rest (PaO2 > 60 mm Hg in room air) after 7 ± 4.1 days.
Afterwards all patients continued to receive heated humidified HFNC without oxygen enrichment (FiO2 21%) at rest to reduce their work of breathing [16]. We report that HFNC also made it easier to perform a relevant rehabilitation plan with respiratory physiotherapy and mobilisation.
CONCLUSIONS
In severe COVID-19 respiratory failure, HFNC is a valid option to support oxygenation in the post-ventilation period. Effectiveness and comfort should be assessed between 2 and 48 hours. Clinical outcomes, oxygenation, and ROX index should be considered to rule out the need for intubation. Further evidence is required for firm conclusions.