Purpose
Brachytherapy (BT) is performed by directly placing the radiation source in or near the tumor site [1]. Brachytherapy plays an essential role in treating cervical cancer. Cervical cancer is a common malignant tumor in females, which seriously threatens women’s health [2]. External beam radiation therapy (EBRT) combined with intra-cavitary brachytherapy (ICBT) is widely used for cervical cancer treatment. However, ICBT provides a lower target coverage when the tumor is bulky, obliterated, or if there is vaginal stenosis or disappearing of vaginal vault, resulting in poorer outcomes [3]. Nonetheless, interstitial brachytherapy (ISBT) combined with ICBT (IC/ISBT) can compensate the disadvantages of ICBT by increasing the dose coverage of clinical target volume (CTV) [4]. The methods for optimizing IC/ISBT mainly consist of graphical optimization and inverse optimization. Inverse optimization methods include inverse planning simulated annealing (IPSA), hybrid inverse planning optimization (HIPO), gradient-based planning optimization (GBPO), GPU-based multi-criteria optimization (gMCO), etc. [5, 6].
Inverse planning simulated annealing reduces the dose to organs at risk (OARs) while increasing the target-dose coverage during cervical cancer treatment with BT [6-8]. Kim et al. [9] and Tinkle et al. [10] found that IPSA has good tolerance and low toxicity, and thus achieves a reasonable local control. Hybrid inverse planning optimization has been widely used in the treatment of cervical cancer and other cancers with BT [11-13]. Trnková et al. [14] concluded that HIPO can eliminate high-dose regions in normal tissue. Fu et al. [15] also showed that the dwell-time distribution produced by HIPO may better match clinical preferences than that produced by IPSA.
Although studies have assessed IPSA and HIPO-based dosimetric perspective, no research has assessed IPSA and HIPO in IC/ISBT for cervical cancer based on radiobiological perspective. Therefore, additional parameters about tumor control probability (TCP) and normal tissue complication probability (NTCP) are expected to make the clinicians to evaluate plans more thoroughly and determine their potential advantages in clinical application. This study aimed to compare IPSA and HIPO from dosimetric and radiobiological perspectives.
Material and methods
Patients
We retrospectively analyzed data of 32 cervical cancer patients who received radical therapy in our hospital from January, 1 to October, 31, 2021. An ethics approval was obtained to include available data in the study. Patients with squamous cell carcinoma were aged between 30 and 77 years. Stages ranged from IB2 to IVA according to the 2018 staging of the Federation of Gynecology and Obstetrics (FIGO) classification [16].
Treatment methods
Patients underwent EBRT and IC/ISBT with or without chemotherapy. Target doses of EBRT and BT were 45 Gy/25 fractions and 30 Gy/5 fractions, respectively. Each IC/ISBT treatment plan for each patient was evaluated separately. BT was performed in an operating room under general anesthesia. A Foley catheter was placed in the bladder, and 7 cc opaque contrast agent was injected in the balloon. Experienced clinicians preliminarily determined the tumor location, size, shape, and invasion using either MRI or CT before BT. A gynecological examination was also conducted before BT. A Fletcher intra-cavity applicator (intra-uterine tube Elekta, part No. 189.739; Stockholm, Sweden) and interstitial needles (range, 4-8 needles; ProGuide plastic needles, Elekta part No. 189.601) were applied for the BT process. A 3D-printed multi-channel vaginal applicator was used for guidance of needle implantation and fixation of the intra-uterine tube. Patients were transported to CT (Brilliance Big Bore CT, produced by Philips, The Netherlands) room to complete localization scan. Scanning range of CT was from the anterior superior iliac ridge to the lower edge of ischial tuberosity. Slice thickness was 3 mm. The image was imported to Oncentra Brachy v. 4.3 treatment planning system (TPS) (Elekta AB). Clinicians delineated HR-CTV and OARs following the recommended standards of the International Commission on Radiation Units (ICRU) (Report No. 89 [17]).
Planning objectives
Treatment plan was performed using Oncentra Brachy TPS. Applicator reconstructions were based on ICRU recommendations [17], and the source stepping size was 5 mm. Offset value of metal uterine tube and interstitial needle value were –0.6 cm and –0.35 cm, respectively [18]. Brachytherapy plan for the first IC/ISBT fraction of each patient was compared. A total of 96 BT plans were created for all patients (each patient had three plans: IPSA, HIPO1, and HIPO2). IPSA employs a fast randomized simulated-annealing algorithm, which takes into account anatomical geometry to optimize source dwell times [19-22]. Optimization process takes less than 1 minute. HIPO combines stochastic simulated-annealing algorithm with a limited-memory Broyden-Fletcher-Goldfarb-Shanno (L-BFGS) deterministic algorithm for three-dimensional (3D) dose-distribution optimization. Manual source position activation and partial catheter optimization are allowed in HIPO [23]. In this study, IPSA, HIPO1, and HIPO2 were completed with the same constraints for anatomical structure of the target area (Table 1). HIPO1 involved maximizing the contribution of uterine tube and locking the uterine tube before optimization, while the uterine tube was not locked and optimized directly under the same initial objectives in HIPO2. Dwell-time deviation constraint (DTDC) and dwell-time gradient restriction (DTGR) of IPSA, HIPO1, and HIPO2 were 0.6. The objective parameters (without graphical optimization) were continuously adjusted to ensure that HR-CTV D90 of all patients reached 6 Gy (difference: less than 0.01 Gy), while OARs’ dose was as low as possible. All plans were calculated for a standardized source strength Sk of 1.6136 × 10-2 Gy.m2.h-1 for iridium-192 (192Ir) source.
Table 1
Optimization objectives used for IPSA, HIPO1, and HIPO2
Dosimetric indexes and data analysis
Dosimetric indexes included isodose lines; HR-CTV D100, V150%, V200%, homogeneity index (HI), and conformity index (CI); D1cc/D2cc for OARs (bladder, rectum, and intestines); and TCP, NTCP, biologically effective dose (BED), and equivalent uniform biologically effective dose (EUBED). DX was defined as the irradiation dose received by the x% relative volume or the × cm3 absolute volume. Vy% was defined as the volume percentage of the prescription dose of y%.
HI and CI calculations
HI [24] was calculated as follows:
CI [25] was calculated as follows:
Where VT,ref is the volume of HR-CTV covered by the prescribed isodose (cm3); VT and Vref are the volume of HR-CTV (cm3) and volume of the prescribed isodose (cm3), respectively.
TCP and NTCP calculations
Tumor dose was not uniform in BT. Niemierko proposed the concept of equivalent uniform dose (EUD) [26] [27], as follows:
Where Vi is the part of the target volume that is irradiated by a dose (Di); Di is the dose received by the voxel volume at i, and a is the parameter that describes the dose-volume effect of tumor or normal tissue.
In this study, a value used was adopted from reference [28, 29] as shown in Table 2. a = +∞, a = –∞, a = 1, and a = 0 indicated maximum dose, minimum dose, average dose, and geometric mean dose, respectively [30]. Local control of the tumor may depend on the minimum dose volume because this is the spot where the survival rate of tumor clones is the highest. Therefore, a should be a large negative value in the tumor. However, a should be a large positive value in normal tissues with tandem structures.
Table 2
Parameters used for TCP and NTCP calculations
| Tissue | Complication | TD50 (Gy) | α/β (Gy) | α | γ50 |
|---|---|---|---|---|---|
| Tumor | – | 70 | 10 | –10 | 3 |
| Bladder | Hematuria | 80 | 3 | 2 | 4 |
| Rectum | Hemorrhage | 80 | 3 | 8.33 | 4 |
| Intestines | Intestinal fistula | 55 | 3 | 6 | 4 |
Niemierko proposed the TCP/NTCP model based on the concept of EUD as follows [27]:
Where TCD50 is the 50% efficiency dose and γ is the parameter that describes the characteristics of the dose-response curve. γ is related to the steepness of the dose-response curve (normalized steepness of the dose-response curve). γ50 is the value of the dose-response curve (γ) at the point of TCP = 0.5 and D = D50. TD50 is the dose required for a 50% probability of complications to an organ caused by radiation on the whole volume.
In the present study, the published code of Gay et al. for calculating TCP/NTCP [29] was applied. These formulas take into account the total dose of radiotherapy. TCP and NTCP were predicted and evaluated based on a single treatment. Nesvacil et al. [31] proposed the formula below, where di represents the dose of voxel i in a single treatment. Di is denoted by (EBRT 45 Gy + IC/ISBT di × 5 fractions):
BED and EUBED calculations
Structures, plans, and dose matrices of single treatments for all patients were extracted from TPS and DICOM. BED using standard linear quadratic (LQ) modes was calculated as follows [32], with each voxel of the dose matrix converted into BED.
Where Di is the total dose delivered to the individual voxel. α/β = 10 Gy for tumor and α/β = 3 Gy for OARs.
The EUBED concept more accurately describes the role of radiobiological effects caused by dose non-uniformity in clinical outcomes. In this study, EUBED was calculated as follows [33]:
Where vi is the fractional normalized volume in the DVH or the ratio Ni/N, Ni is the number of voxels receiving the same dose, and α is 0.3 [33].
Statistical analysis
SPSS v. 26.0 software (IBM, Armonk, NY, USA) was used for statistical analyses. Data were presented as mean ± standard deviation (x̄ ± S). Evaluation parameters of each group, which conformed to a normal distribution as determined through normality testing, were conducted using a matched samples t-test. Test methods of TCP and NTCP were different because dosimetric differences did not follow a linear relationship, and the data were not normally distributed. A non-parametric Friedman test was then conducted [34, 35]. However, non-parametric Wilcoxon rank test was applied for post-hoc multiple comparisons in cases when the assumptions in Friedman test were rejected (p < 0.05). P-value < 0.05 indicated statistically significant differences.
Results
Isodose line
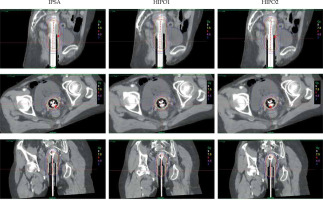
Figure 1 shows an image of a representative case from the patient cohort. The 9 Gy isodose line (high-dose region) of HIPO1 was smoother than that of IPSA and HIPO2, especially in the cervix region.
Target and OARs doses
The target volumes in the 32 patients were 43-156 cm3 (median, 93 cm3). The difference in target dose among the three techniques is shown in Table 3. D100 had no remarkable difference between HIPO1 and HIPO2 (p > 0.05). V150% and V200% were higher in HIPO1 than in IPSA and HIPO2 (Figure 2) (p < 0.05). However, V150% and V200% of IPSA and HIPO2 were similar (p > 0.05). CI was higher in HIPO2 than those in IPSA and HIPO1 by 3.80% and 2.50%, respectively (p < 0.002). However, HI was lower in HIPO1 than in HIPO2 and IPSA by about 2.50-4.88%.
Table 3
Comparison of physical parameters of IPSA, HIPO1, and HIPO2 plans
Fig. 2
Main dosimetric parameters of IPSA, HIPO1, and HIPO2 (D2cc of the bladder, rectum, and intestines; D100, V150%, V200%, HI, and CI) were analyzed via box method. Asterisk (*) indicates that the corresponding p-value is between 0.01 and 0.05. Two asterisks (**) indicate that the corresponding p-value is between 0.001 and 0.01. Three asterisks (***) indicate that the corresponding p-value is less than or equal to 0.001
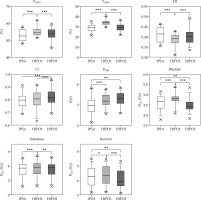
D1cc and D2cc of OARs were highest in HIPO1 than those in IPSA and HIPO2. The doses of D2cc to the bladder and rectum were lowest in HIPO2 than in of IPSA and HIPO1. Also, the average values of D1cc of OARs were similar between IPSA and HIPO2.
Results of radiobiological model
EUBED: The comparison average results of EUBEDs among the three plans are shown in Table 4. IPSA had the lowest EUBED of HR-CTV (12.27 ±0.28 Gy) than in HIPO1 and HIPO2. EUBED in HR-CTV was similar between HIPO1 and HIPO2 (p > 0.05). HIPO2 had the lowest EUBEDs of the bladder and rectum compared with IPSA and HIPO1. Furthermore, EUBEDs of the bladder, rectum, and intestines were highest in HIPO1 than in IPSA and HIPO2.
Table 4
Comparison of EUBED among IPSA, HIPO1, and HIPO2
TCP and NTCP: TCPs found no remarkable difference between IPSA and HIPO1 (Table 5). NTCPs of OARs were lower in IPSA than in HIPO1 by 4.17% (bladder), 24.65% (rectum), and 8.55% (intestines). Moreover, TCPs found no remarkable difference between IPSA and HIPO2. NTCP of the bladder was lower in HIPO2 than in IPSA by 13.04%. TCP was higher in HIPO2 compared with HIPO1 by 0.23%. NTCPs of the bladder and rectum were lower in HIPO2 than in HIPO1 by 16.67% and 42.33%, respectively.
Table 5
Comparison of TCP and NTCP among IPSA, HIPO1, and HIPO2 (median, quartile)
Discussion
Brachytherapy plays an essential role in cervical cancer treatment, and IPSA is widely used in BT. However, HIPO has also been introduced into BT [36]. DTDC and DTGR are used to modulate the dwell-time distribution in IPSA and HIPO, respectively. Furthermore, IPSA and HIPO have different operating principles [37, 38]. For example, the DTDC parameter in IPSA defines an upper limit of dwell time, which controls the dwell-time change between adjacent dwell locations in each catheter while the DTGR parameter of HIPO is a dwell-time-gradient constraint that limits the large time fluctuation in the adjoining dwell location. A previous study suggested that the DTDC value should be about 0.6 in ICBT if OARs’ doses are limited for patients with radical cervical cancer [39]. Although DTDC and DTGR have different operating principles, the range of both DTDC and DTGR is 0-1. Therefore, taking the same value in the range of 0-1 has similar modification effect; therefore, the DTDC and DTGR values were both set at 0.6 in this study.
Herein, various radiobiological metrics were established to assess the correlation between radiation dose and biological effects for better clinical practice. EUD can be used to calculate TCP and NTCP of non-uniform dose distribution. Other models, such as Lyman-Kutcher-Burman (LKB) [40] or relative seriality (RS) [41] models have the proper formulations to accommodate this need as well. In this study, Niemierko’s model was selected because it is simple and convenient for calculation, and is recommended for BT in the AAPM Report 137. Chow et al. [42] retrospectively analyzed the range of fixed parameters used in BED model, which are caused by individual differences in patients. Several other researchers studying squamous cell carcinoma have also reported the range of these parameters. In this study, α and α/β were 0.3 Gy-1 (0.06 ~ 0.74 Gy-1) and 10 Gy (5.9 ~ 76.9 Gy), respectively. Furthermore, the median values were used for data comparison since the study was conducted based on a single treatment. The α/β ratio for all OARs was 3 Gy.
The current study has some limitations. This study mainly aimed to compare the differences of physical and radiobiological doses between different optimization methods in a single IC/ISBT fraction. However, all EBRT and the other four BTs in the total treatment were unified and simplified. Also, the adjustment of optimization parameters is subjective, and it largely depends on dosimetrist/physicist experience.
Human biological information is complex and diverse. Therefore, a comparative trial should be further carried out to obtain a higher level of clinical evidence. Although the statistical results can provide a reference for clinical radiotherapy workers, a more in-depth research is needed to solve the influence on those radiobiological factors.
Conclusions
Although the two inverse optimization algorithms meet clinical needs in cervical cancer treatment through combined ICBT/ISBT, HIPO2 (with an unlocked uterine tube) provides better dose conformability and lower NTCP. Therefore, HIPO2 is recommended as an optimization algorithm in IC/ISBT of cervical cancer.