Globally the number of geriatric patients undergoing routine/lifesaving surgeries is on the rise with the advancement of the healthcare system and improved life expectancy. However, postoperative cognitive dysfunction (POCD) in the elderly, either in the form of disordered thinking and/or impaired higher mental functions following surgery and anaesthesia, is a serious concern due to increased stress to the patient as well as the caregiver, as well as associated morbidity. The overall incidence of POCD is 25.8% at one week, 9.9% after 3 months, and advanced age has been attributed as a significant risk factor for it [1, 2].
Although POCD is clinically described as a deviation from normal cognition, there is no consensus regarding the extent of the variation. While the International Classification of Diseases (ICD-10) defines it as “mild cognitive impairment”, the Diagnostic and Statistical Manual of Diseases-5 (DSM-5) does not acknowledge it. In 2018, a nomenclature consensus working group devised recommendations and guidelines to define the timing of various cognitive deviations in the perioperative period [3].
Since neuroinflammation, neuronal cytotoxi-city, and apoptosis due to exposure to anaesthetic agents have been potentially implicated in the pathophysiology of POCD, the role of neuroprotectants has become prominent and is an active area of interest.
Lidocaine and dexmedetomidine have recently become prominent due to their increased use in anaesthetic and critical care practice for sedation and analgesia. Both lidocaine and dexmedetomidine suppress the inflammatory markers that are usually ele-vated in the post-surgical period, as well as neuron-specific markers of inflammation like neuron specific enolase (NSE) and S-100β, compared to controls [4–6].
Dexmedetomidine, a dextro-enantiomer of medetomidine and a highly selective α2 adrenoreceptor agonist, has sedative, analgesic, and sympatholytic properties, thereby reducing the requirement for anaesthetic agents. α2 adrenergic receptors are abundant in the dorsal noradrenergic bundles, locus coeruleus, and frontal lobe, which are crucial for cognitive function, memory, learning, and selective attention. Zhang et al. [7] reported that a 0.5 μg kg–1 loading dose over 10 minutes followed by an infusion dose of 0.5 μg kg–1 h–1 in elderly patients undergoing laparoscopic surgery under general anaesthesia for colorectal cancer provided neuroprotection regarding the reduced incidence of POCD and neuro-inflammatory marker levels. A meta-analysis also found that dexmedetomidine during surgery suppressed inflammatory cytokines, particularly IL-1 and IL-6, perioperatively and helped preserve cognitive functions in the elderly [8].
Lidocaine is a 1b class of anti-arrhythmic, sodium ion channel blocker that acts as a neuro-protectant by reducing the cerebral metabolic rate [9]. Animal studies have found that it decreases the release of ischaemic excitotoxin by reducing the transmembrane ion shift in the brain [10]. Wang et al. [11] showed that lidocaine prevented the occurrence of POCD on day 9 following coronary bypass surgery on cardiopulmonary bypass (CPB). Another study on elderly patients undergoing spine surgery reported that the lidocaine group had better Mini-Mental State Examination (MMSE) scores in comparison to the control group, with markedly reduced levels of IL-6 and S-100β [4].
Neuroinflammation, protein deposition in neurons, and neuronal damage are often attributed to POCD [12]. Factors like hypoperfusion and systemic inflammation increase the risk [13]. Elevated levels of interleukins (IL-6), cortisol, and S-100β indicate inflammation and neuronal damage. IL-1 and TNF-a affect neuronal metabolism and induce microgliosis, respectively [13–17].
Because there is no comparative evaluation of both drugs for preventing POCD, this study aims to compare the relative efficacy of their potential neuro-protective action in terms of the incidence of POCD in elderly patients undergoing open abdominal surgery under general anaesthesia when administered intraoperatively. The primary objective was to compare the effect of intraoperative administration of dexmedetomidine with lidocaine on the incidence of POCD in elderly patients on postoperative day 3, with the secondary objective to correlate the incidence of postoperative cognitive decline with changes in levels of serum IL-1, IL-6, TNF-a, amyloid-β, and S100.
METHODS
This single-centre study was conducted after acquiring institutional Ethics Committee approval (IECPG-221/28.06.2018), enlisting in the Clinical Trials Registry of India (CTRI/2018/08/015358) prospectively, and written informed consent in 64 patients with ASA physical status I or II, elderly (age 60–80 years), undergoing open abdominal surgery under general anaesthesia, with an anticipated duration of more than 2 hours. Elderly patients with preoperative Mini Mental State Examination Score below 24, electrolyte imbalance, bradycardia (HR below 45 beats per minute), or history of alcohol or any substance abuse, seizures, cerebrovascular accidents or intracranial surgeries, psychotic disorders, dementia, any disorder affecting cognition and higher mental functions, disease associated with systemic or central nervous system inflammation, patients with difficulty in hearing/speech/unable to read even with assisted vision, or with refractory intraoperative hypotension (mean arterial blood pressure [MAP] below 60 mmHg) causing discontinuation of intervention agent were excluded.
The patients were randomly assigned to receive either lidocaine (n = 32) or dexmedetomidine (n = 32) using http://www.randomizer.org and simple randomization. A serially numbered opaque sealed envelope method was used to conceal random allo-cation. The primary investigator, participants, and data analysts were blinded to group allocation.
Neuropsychological assessment
All the enrolled patients underwent the following neuropsychological tests along with routine preoperative assessment on the day before the surgery and on postoperative day 3:
Mini Mental State Examination (MMSE): This is a screening tool to assess an individual’s cognitive status. It consists of a set of 30 questions in a sequential format, with a total score of 30. A significant cognitive deficit is indicated with a score below 24. However, it does not assess the executive functions of cognition.
Montreal Cognitive Assessment (MoCA): This was designed by Naseridine to assess mild cognitive impairment in 1996. The format is the same as MMSE with 2 distinct differences, i.e. the time-bound and graded assessment of each domain and the assessment of additional cognitive domains of abstract thought and executive functions. It is a single-page, 30-point test done in approximately 10 minutes to assess attention, memory, executive functions, visuospatial abilities, abstraction, delayed recall, and orientation. While MMSE was found to be very efficient in detecting pre-existing cognitive impairment [18], the sensitivity of MoCA was significantly higher (90%) than that of MMSE (18%) in the detection of mild cognitive impairment, with comparable specificity (100% and 87%, respectively) with a cut-off score of 26 [19].
Stroop test : This test assesses cognitive flexibility and inhibitory control by measuring the interfe-rence between automatic and controlled processes. Participants are asked to name the ink colour in which words are printed, while the words represent conflicting colour names.
Porteus Maze test: This involves navigating a virtual maze to locate hidden objects while facing different environmental conditions and challenges, to assess spatial cognition, strategy development, and memory.
Trail making test: This test evaluates attention, executive function, and visual-motor coordination by measuring cognitive flexibility, visual scanning, and mental processing speed. Participants are instructed to connect a series of numbers or letters in ascending order while avoiding a predetermined pattern.
All these tests were conducted by a psychiatrist, and a reduction of more than 20% in the baseline scores of any 2 tests was defined as POCD.
Biomarker assessment
Pre-operatively, after securing an intravenous line, and on the postoperative day 3, two blood samples were collected in clot activator and serum gel separator vacutainer; serum samples were obtained by centrifugation on the same day of collection, stored at –80°C, and assayed simultaneously for measurement of IL-1, IL-6, TNF-a, amyloid-β, and S100 using the following sandwich Enzyme Linked Immunosorbent Assay (ELISA):
IL-1 (eBioscience Human il-1 beta) – BMS224/2/BMS224/2TEN Platinum ELISA Kit.
IL-6 (Elabscience, Human IL-6) – E-EL-H0102 ELISA Kit.
TNF-α (Elabscience, Human TNF-a) – E-EL-H0109 ELISA Kit.
S-100β (Thermo Scientific, Human S100A) – EHS100B ELISA Kit.
The biotechnologist was blinded to the patient’s history and anaesthesia.
Intraoperative management
A thoracic or lumbar epidural catheter was placed in all the patients before induction, according to the surgery and preference of the attending anaesthesiologist.
The lidocaine group of patients received a bolus dose of the lidocaine (1 mg kg–1) over 10 minutes before induction, followed by an infusion (1.5 mg kg–1 h –1), which was discontinued at the skin closure.
Similarly, the dexmedetomidine group of patients received a bolus dose of dexmedetomidine (0.5 μg kg–1) over 10 minutes before induction, followed by an infusion (0.5 μg kg–1 h–1), which was also discontinued at the skin closure.
All the patients were induced with propofol 1–2 mg kg–1, fentanyl 1–2 μg kg–1. The tracheal intubation with an appropriately sized, cuffed endotracheal tube was facilitated with atracurium of 0.5 mg kg–1. Volume-controlled mode of ventilation with a tidal volume of 6 to 8 mL kg-1 and positive end-expiratory pressure (PEEP) at 5 cm of water was used with an end tidal CO2 targeting around 35 to 38.
Anaesthesia was maintained with oxygen, air, and isoflurane in all the patients, maintaining a minimum alveolar concentration (MAC) titrated to maintain a bispectral index (BIS) of 50–60. The patients were also given atracurium boluses at 25% of the intubating dose. An intravenous fentanyl bolus of 0.5 μg kg–1 was administered when the heart rate increased by more than 20% from the baseline. Intravenous (IV) paracetamol (15 mg kg–1) and ketorolac (0.5 mg kg–1) was also administered for intra-operative analgesia. Ringer lactate, Plasma-Lyte A, or Sterofundin were used for maintenance fluid at the discretion of the treating anaesthetist according to the Holliday-Segar formula (100 mL kg–1 for the initial 10 kg of weight, 50 mL kg–1 for the next 10 kg of weight, 20 mL kg–1 for the successive weight).
IV ondansetron (0.1 mg kg–1) was given 20 minutes before closure of the incision for postoperative nausea and vomiting prophylaxis. The infusions of lidocaine or dexmedetomidine were discontinued at the skin closure, and the residual neuromuscular blockade was reversed using neostigmine (50 μg kg–1) and glycopyrrolate (10 μg kg–1).
Postoperative management
After shifting the patient to the PACU, the sedation level was assessed as per the Ramsay Sedation Scale (RAS) at one hour, and the VAS was evaluated hourly. The postoperative analgesia was achieved with epidural morphine 50 μg kg–1 at 12-hour intervals. The rescue analgesia was intravenous fentanyl boluses (0.5 μg kg–1) if the VAS > 4.
Sample size estimation
In a retrospective study by Chen et al. [20], the dexmedetomidine group showed an incidence of POCD of 9.20% compared to the control group’s 21.31%. The postoperative mean MMSE score was 25.86, and the standard deviation was 4.10 in the dexmedetomidine group. We hypothesize that with the use of lidocaine, around 15% improvement in postoperative MMSE score (estimated mean value of 29.7) can be expected. With a power of 90% and a probability of alpha error of 5%, at least 24 patients would be required in each group to detect a statistically significant difference. Considering a dropout score of 20%, 60 patients were required. The sample size was estimated with STATA 12.0 SE for Mac OS (Stata Corp. 2011. STATA STATISTICAL SOFTWARE: Release 12. College Station, TX: Stata Corp LP).
Statistical analysis
Normally distributed continuous variables were expressed as mean and standard deviation (SD), non-normally distributed variables and categorical variables as the median and inter-quartile range (IQR), and binary variables were described in absolute numbers and proportions.
The percentage change in score was calculated as follows: Percent change in score = (Postoperative score – Preoperative score)/Preoperative score × 100. The Mann-Whitney U test was applied to compare categorical and non-normally distributed continuous variables, and binary variables were compared using Fisher’s exact test. A P-value of less than 0.05 was considered statistically significant for all tests.
RESULTS
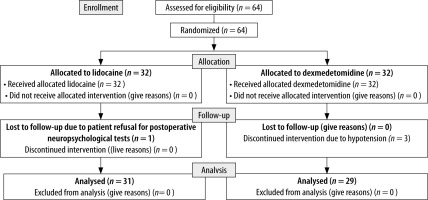
Sixty-four patients were assessed for eligibility, of whom 60 were analysed in this trial. Thirty-two patients were allotted to each group as per simple randomisation. One patient in the lidocaine group refused postop neuropsychological assessment, and in the dexmedetomidine group the infusion was discontinued in 3 patients due to hypotension (Figure 1).
Demographic parameters, duration of anaesthesia and exposure to anaesthetic gases, intraoperative use of opioids, and blood transfusion in both arms were comparable. The incidence of bradycardia was greater with dexmedetomidine. Nine patients (29.03%) in the lidocaine group and 7 patients (24.1%) in the dexmedetomidine group showed an increase of ≥ 20% in the test scores in > 2 tests and satisfied the predetermined criteria for diagnosis of POCD on postoperative day 3 (Table 1).
TABLE 1
Comparative evaluation of demography, intraoperative. and post parameters and outcome with lidocaine and dexmedetomidine
Both groups had no significant difference in percentage change in all the postoperative neuropsychological assessment scores. IL-1 rose by 449% and 202% with lidocaine and dexmedetomidine, respectively, on postoperative day 3 (P = 0.03). Even though there was a comparable rise in the levels of all the biomarkers, the changes for TNF-a, IL-6, and S-100β were statistically comparable in both groups (Table 2).
TABLE 2
Comparative evaluation of cognitive scores and serum biomarkers with lidocaine and dexmedetomidine
The incidence of hypotension, its duration, and the number of episodes all were found to substantially increase the risk of developing POCD. All the biomarkers showed a rise in serum concentrations irrespective of the cognitive decline. There was no statistically significant difference despite elevated values of some interleukins in patients with POCD (Table 3).
TABLE 3
Distribution of demographic, intraoperative, and postoperative parameters and different biomarkers by occurrence of POCD
No significant correlation was found between the different neuropsychological test scores and neuroinflammatory biomarkers (Table 4) and between their percentage changes (Table 5).
TABLE 4
Correlation between the neuropsychological test scores and neuroinflammatory biomarkers on postoperative day 3 (Pearson’s correlation coefficient)
TABLE 5
Correlation between the percent change in neuropsychological test scores and neuroinflammatory biomarkers on postoperative day 3
DISCUSSION
The incidence of POCD and changes in levels of IL-6, TNF-a, and S-100β did not vary significantly between the 2 interventional arms in the present study. Among the other observed variables, intraoperative haemodynamic changes were significant in the dexmedetomidine group. Intraoperative hypotension was found to be a risk factor for developing POCD.
The incidence of POCD in the lidocaine group (29.03%) was lower compared to the observational study done in the Indian population by Shiraboina et al. [21] (incidence of 37.64%). Wang et al. [11] recruited 118 patients undergoing coronary artery bypass surgery into a trial with continuous intraoperative lidocaine infusion (4 mg kg–1) and found the incidence of POCD to be 10%.
Among the neuropsychological tests, MMSE is a standard; a score of below 25 was excluded in this study because evidence for a preoperative cognitive deficit in the study by Chen et al. [20] MMSE was the sole test used to determine the incidence of POCD on day 1. The median preoperative MMSE scores were comparable with our study, but the postoperative scores showed a greater decline (3 points compared to 1, respectively). This diffe-rence could be due to the time point of evaluation, which was not specified and may significantly affect test scores due to residual sedation in the imme-diate postoperative period.
The biomarkers of neuroinflammation act as a surrogate for the underlying mechanisms that may provide a physiologic basis for the claim of protection against cognitive decline by the intervention agents used in our study. The markers of inflammation rose in serum in the postoperative period in both groups. Kui Chen et al. [4] analysed the biomarker levels at postoperative days 1, 3, and 7. They found TNF-a, IL-6, NSE, and S-100β to have risen in the serum at day 3 in both the placebo and lidocaine groups. This is consistent with the finding in our study, but the absolute values in serum show considerable variability, probably due to the diffe-rence in demographics. Another finding of this study was a significantly lesser rise in IL-6 and S-100β on postoperative day 3 in the lidocaine group compared to the placebo group (P < 0.05).
The retrospective study by Chen et al. [4] found a considerable reduction in IL-6 and TNF-a serum levels in the dexmedetomidine group compared to the placebo group (P < 0.05). A meta-analysis reported that the use of dexmedetomidine during surgery suppressed inflammatory cytokines perioperatively and helped in preserving the cognitive functions in the elderly, especially IL-6 [22].
Kotekar et al. [23] studied the Indian population and found age as well as female gender to indicate a higher risk for the development of POCD. The age and gender disparity was not evident in our patients. ISPOCDII study found general anaesthesia, and duration of administration of general anaesthesia, to be risk factors [24]. Although the median duration of anaesthesia exposure was higher in the POCD group by 20 minutes, it was not statistically significant. This can be explained by mainly gynaecological surgeries being assessed in our study, with similar operating time frames. Our study group had a median BMI of 22.5; hence, the effect of obesity and body mass on POCD, due to its exaggerated inflammatory state, was not evident in our study. The educational status of patients was similar among the 2 groups.
Hypotension was a known and anticipated complication of one of our intervention agents. However, its incidence did not differ between the 2 groups. The number of patients having intraope-rative complications of hypotension (P = 0.09), hypotensive episodes (P = 0.006), and duration (P = 0.07) were found to be significant in those who developed POCD. Thus, we hypothesize that hypotension leading to reduced transient cerebral perfusion and hypoxia could be causative for the occurrence of POCD. Shiraboina et al. [21] found blood transfusion to be the single most prominent risk factor in cardiac surgery patients. Blood loss and blood transfusion were notably greater in the patients who developed POCD. In our study, the pain management was multimodal and involved epidural and intravenous opioids and non-steroidal anti-inflammatory drugs (NSAIDs), as well as timely VAS-guided epidural doses of opioids with an overarching cover of NSAIDs in the postoperative period; therefore, postoperative pain did not play a significant role in our patients for the development of POCD, with comparable pain scores between both intervention arms.
LIMITATIONS
Lidocaine and dexmedetomidine plasma concentrations were not assessed. The definition of POCD as per the Consensus Group also includes it being a time-sensitive phenomenon, but our follow-up was only until postoperative day 3. Most of the patients were diagnosed with a malignant pathology, which may have caused a generalized inflammatory state and thus been a confounding factor for the measured serum interleukins. The ge-neralizability is also limited for being a single-centre study.
CONCLUSIONS
There is no difference in the incidence of POCD in patients receiving either lidocaine or dexmedetomidine during the intra-operative period. The gene-ralized better anti-inflammatory effect of dexmedetomidine in terms of lesser rise in postoperative IL-1 compared to lidocaine needs to be explored.