Worldwide, breast cancer remains the most frequent malignancy among women [1]. In Egypt, it accounts for 38.8% of female cancers [2, 3]. The first-line surgical treatment for breast cancer is modified radical mastectomy (MRM), which involves surgical excision of the whole breast and the axillary lymph nodes [4].
Acute postoperative pain is reported in around 40% of breast cancer patients, indicating that post-operative pain treatment is insufficient [5]. In addi-tion, acute postoperative pain could reduce the quality of life by increasing the probability of postmastectomy pain syndrome [6].
Another significant issue is the high prevalence of breast cancer-related postoperative nausea and vomiting (PONV) [7]. These unpleasant sensations may result in significant surgical complications, such as poor sleep and delayed spontaneous ambulation [8].
Regional anaesthesia has resulted in better acute pain control and a reduction in chronic pain [9]. Hence, thoracic epidural [10], thoracic paravertebral [11], intercostal nerve [12], and pectoral nerve [13] blocks are among the most effective therapeutic options for addressing these issues in MRM patients; however, each strategy has its own benefits and drawbacks. As a result, ultrasound (US) guidance has been introduced to assist regional nerve blocking procedures [14].
The pectoral nerve (PECS) block and the serratus anterior plane (SAP) block are considered effective blocks for post-mastectomy analgesia [15]. Blanco and his colleagues [16, 17] described the pectoral nerve block as interfascial plane block. In PECS I block, a local anaesthetic is injected between the pectoralis major and minor muscles, while in PECS II block, a local anaesthetic is injected above the serratus anterior muscle just at the third rib. These techniques target the long thoracic nerve as well as the pectoral, intercostobrachial, and inter-costal III, IV, V, and VI nerves.
The serratus anterior plane block is applied above the serratus anterior muscle, between it and the latissimus dorsi. The nerve to the serratus anterior, the anterolateral branch of the thoracic intercostal nerves T2–T8/9, and the thoracodorsal nerve are anaesthetized by SAP block. A serratus-intercostal fascial plane block is performed by an SAP block below the serratus anterior muscle. The lateral and anterior cutaneous branches of the intercostal nerves T2–T8/9 are anaesthetized by the SAP block. Compared to other forms of thoracic plane blocking, the local anaesthetic is injected into a side that is more dorsal (e.g., interpectoral plane blockade and pectoserratus plane blockade). Because of this, SAP block can anaesthetize more intercostal nerves and more specifically reach the thoracic nerves [15, 18, 19].
In studies comparing post-mastectomy acute pain control, the thoracic paravertebral block was outperformed by the PECS block [20], and the serratus plane block was reported to be less effective than the thoracic paravertebral block [21]. Hence, this study aimed to compare the quality of analgesia produced with the US-guided PECS II block versus the SAP block in patients undergoing MRM.
METHODS
Ethical considerations
The study was carried out following approval by the Ethics Committee of the Faculty of Medicine, Suez Canal University, Egypt (Reference number: 4737). This trial was registered at ClinicalTrials.gov (Trial ID: NCT05224310). Written informed consent was obtained from each participant after explanation of the purpose and procedures of the study. All participants’ data were kept confidential.
Study design, setting, and date
This single-blinded, parallel-group, randomised trial was conducted at Suez Canal University Hospital, Egypt between April 2022 and June 2022.
Eligibility criteria
The present study included 50 adult female patients who were American Society of Anaesthesiologists (ASA) physical status I or II and were scheduled for MRM under general anaesthesia.
We excluded patients who had a history of allergy to the drugs used, were on chronic pain medications or analgesics, were pregnant or breastfeeding, and those with a tendency for bleeding, thrombocytopenia, chronic liver or kidney disease, and apparent anatomical abnormalities or infection in the serratus region.
Randomization, allocation concealment, and blinding
Fifty female patients were randomly allocated to two groups (25 patients each). The PECS II group underwent US-guided pectoral nerve block, and the SAP group underwent US-guided serratus plane block. Randomization and allocation concealment were performed using the sequentially numbered, opaque, sealed envelopes method [22]. The envelopes were impermeable to intense light and the allocation sequence was concealed from the physician assessing and enrolling participants. To prevent subversion of the allocation sequence, the name and hospital admission number of the participant were written on the envelope. The corresponding envelope was opened only after the enrolled participant completed all baseline assessments and it was time to allocate the intervention. The study participants were kept blinded to the allocation.
Interventions
Preoperative management
Preoperatively, patients were instructed how to evaluate their pain intensity using the visual analogue pain scale (VAS) and how to use the patient controlled analgesia (PCA) device.
All patients were subjected to detailed history taking and thorough physical examination, especially the serratus area. Routine preoperative investigations were performed including complete blood count, prothrombin time, partial thromboplastin time, international normalised ratio, liver function tests, serum creatinine, random blood sugar, and HbA1c. Chest X-ray and 12-lead ECG were done for post-menopausal patients as well as patients with cardio-vascular diseases.
Intraoperative management
Upon arrival at the operating room, basic monitoring was applied for the heart rate (HR), non-invasive blood pressure, oxygen saturation, and ECG. On the contralateral side of the surgery, a 20-gauge or wider intravenous (IV) line was secured. To induce general anaesthesia, fentanyl (1 µg kg-1) and propofol (2 mg kg-1) were administered IV. Cisatracurium (0.15 mg kg-1) was given to facilitate endotracheal intubation. The lungs were mechanically ventilated to sustain a 35–40 mmHg end-tidal CO2 level. Anaesthesia was maintained with isoflurane at end-tidal 1.3% (~1.2 MAC) in 50% oxygen/a ir mixture by controlled mechanical ventilation, keeping the end-tidal CO2 at 35–40 mmHg. If the mean arterial pressure (MAP) and/or the HR increased by 20% from the preoperative baseline, further bolus doses of fentanyl 0.5 µg kg-1 were administered. Hypotension, defined as reduction of blood pressure more than 20% of baseline, was managed by 6 mg of ephedrine intravenously. Bradycardia defined as a decrease of heart rate more than 20% of baseline was managed by incremental doses of 0.5 mg of atropine intravenously. All the blocks were performed in an aseptic setting with the patients being observed.
Block performance
US-guided PECS II block was performed on the same side as the surgery. The patient lay supine with the ipsilateral arm abducted and externally rotated and the elbow flexed at 90 degrees. The high-frequency probe was placed transversely between the clavicle medially and above and the shoulder joint laterally. After identifying the pectoralis major and minor muscles and the plane between them, the probe was angled caudally to look for the pulsating pectoral branch of the thoracoacromial artery; if it was not found, the probe was pushed 1–2 cm caudally and medially. In a caudal tilt, within a biconvex space, the artery was recognised. After that, the B-Braun Stimpulex 22G X 100 mm needle was pushed in an in-plane approach to the artery’s location. After that, 10 mL of 0.25% bupivacaine was administered. The probe was then moved laterally and caudally towards the anterior axillary fold until the serratus muscle appeared beneath the pectoralis minor muscle attached to the underlying ribs. The third and fourth ribs were detected. The needle targeted the plane between the serratus and the third rib, followed by negative aspiration into the fascial plane then injection of 20 mL of 0.25% bupivacaine.
In the serratus plane block group, the patient lay in the supine position, and US-guided serratus block was performed. The ribs were then counted, and the high-frequency probe was placed over the fourth rib in the mid-axillary line in a sagittal plane. When the ribs, pleura, latissimus dorsi muscle, teres major muscle, and overlaying serratus muscle were detected, a B-Braun Stimpulex 22G X 100 mm needle was pushed cephalad in until the needle tip reached the fourth rib. After that, 30 mL of 0.25% bupivacaine was injected deep into the serratus muscle.
In both blocking approaches, the 13–6 MHz transducer (FUJIFILM SonoSite, Inc. Bothell, WA 98021 USA) was used. The time spent from holding the ultrasonography probe until the needle was withdrawn after injection was recorded. The skin incision was performed 20 minutes after giving the block.
Postoperative management
All patients were transported to the post- anaesthesia care unit (PACU) after extubation and being fully awake and were hospitalised for at least 24 hours. In the immediate postoperative period, the haemodynamic parameters (respiratory rate, HR, and MAP) were recorded every 30 minutes for the first two hours then every two hours for the remaining 22 hours. The first request for analgesia was defined as the time from completion of the block to the time of the first request for analgesia. The duration of sensory block and the intensity of pain were assessed immediately postoperatively and after 2, 4, 8, 16, and 24 hours by the visual analogue scale (VAS) [23]. The VAS ranges from 0, indicating no pain, to 10, indicating severe intolerable pain, with variable degrees of ascending pain in between. The VAS was assessed at rest by an anaesthesia resident who was blinded to the performed technique. Postoperative morphine analgesia was given by PCA; once the VAS was more than or equal to four, the patient pressed a button on the PCA pump (Graseby 3300 PUMP; Smith Medical International, Ashford, Kent, UK), which was programmed to deliver a bolus of 1 mL (1 mg of morphine), with 20 minutes lockout interval, and a maximum dose of 4 mg in 4 hours. Paracetamol 1 g infusion was given postoperatively every 6 hours for 24 hours. The total analgesic consumption and surgeon satisfaction score were recorded for the first 24 hours (0, not satisfied; 1, satisfied). The rate and severity of nausea and vomiting were assessed according to a four-point scale (1, indicating no nausea or vomiting; 2, mild; 3, moderate; and 4, severe).
Outcomes
The primary outcome was the time to first anal-gesic request. The secondary outcomes included the postoperative pain that was assessed using the VAS score immediately postoperatively and during the first 24 hours, the total intraoperative and postoperative analgesic consumption during the first 24 hours as well as the total procedure time, surgeon satisfaction, intraoperative and postoperative haemodynamic parameters, and postoperative nausea and vomiting. Furthermore, the correlation between VAS and different factors was studied to detect the possible risk factors for postoperative pain in breast surgery.
Sample size
According to Shokri and Kasem [24], an estimated 25 patients per group would be needed to provide 90% power for independent populations, assuming a 20% increase or decrease in time to first analgesic request (632.48 or 421.9 min respectively) in the PECS II group (corresponding to a mean duration of postoperative pain relief following breast surgery of 527.4 minutes, with a standard deviation of 105.4, with a unilateral a of 0.05).
Statistical analysis
For quantitative data, the Shapiro-Wilk test for normality was performed. Normally or continuously distributed data were summarised as mean ± standard deviation (SD), and the studied groups were compared using Student’s unpaired t-test. All the haemodynamic parameters follow the normal distribution, were presented as mean (SD), and were compared by independent t-test (at each specific time point). Nonparametric data such as VAS score were presented as median and interquartile range (IQR) and were compared using Mann-Whitney U test (at each specific time point). Qualitative data were summarised as frequencies, and associations were tested using Pearson’s c2 test or Fisher’s exact test. Spearman correlation analysis was used to evaluate the correlation between age and VAS score. All tests were two-tailed. A P-value < 0.05 was considered significant.
RESULTS
Fifty-five patients were recruited; 2 patients refused to participate, and 3 patients were excluded due to coagulation disorders. Fifty patients were enrolled in the study and were randomly allocated to two groups (25 patients each) (Figure 1).
Table 1 reveals no significant differences between the two groups regarding ASA status, presence of chronic diseases, and duration of surgery, but we found significant differences between the studied groups in both age and body mass index.
TABLE 1
Demographic and baseline patients’ characteristics
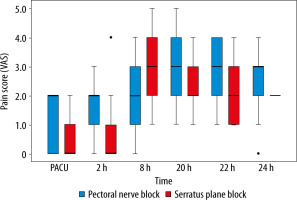
As shown in Table 2, the mean time to the first rescue analgesia within the first 24 hours was significantly longer in the SAP group than the PECS II group with a mean difference of 332 min (95% CI: 90.2–574.5, P = 0.009); furthermore, the patients’ need for analgesia was significantly lower in the SAP group with relative risk 1.76 (95% CI: 1.19–2.62, P = 0.002) than the PECS II group. The total consumption of IV morphine was significantly less for patients in the SAP group compared to that of the PECS II group, with a mean difference of 1.36 (95% CI: 0.33–0.69, P < 0.05). However, intraoperative fentanyl requirements were non-significantly different between the groups (P = 0.8). The total time for performing the block was significantly lower in the PECS II group than the SAP group, with a mean difference of –2 (95% CI: 2.96–0.95, P < 0.05). Surgeons’ satisfaction scores were non-significantly different between the two groups (P = 0.7). The incidence of PONV was higher in the PECS II group than the SAP group, but the difference did not reach statistical significance, with a mean difference of 2 (95% CI: 0.5–7.1, P = 0.46). Postoperatively, the VAS scores in the SAP group were considerably lower compared to the PECS II group at the time of admission to the PACU, as well as at 2, 8, 20, 22, and 24 hours (P < 0.05) (Figure 2).
TABLE 2
Postoperative analgesic consumption, postoperative visual analogue scale, surgeons’ satisfaction, and postoperative complications
FIGURE 2
Box plots of postoperative pain scales (VAS) in patients who received pectoral nerve block II and serratus plane block over the first 24 hours. The horizontal coloured line in each box represents the median value, the outer margins of the box represent the interquartile range, and the circles represent outliers. All P < 0.05
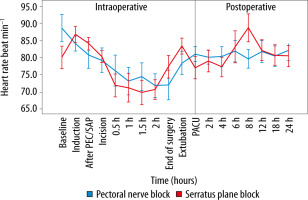
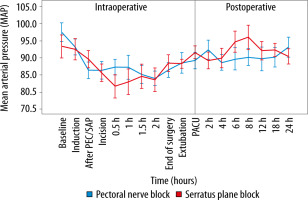
Spearman correlation analysis showed a non-significant correlation between age and VAS score at the PACU (P = 0.25), and age and VAS score at 24 hours postoperatively (P = 0.31). The haemodynamic parameters were comparable, most of the time, in the two groups (Figures 3 and 4). At the conclusion of surgery, when patients were extubated, and eight hours after surgery, the PECS group’s HR was significantly lower. Significant intraoperative MAP reductions occurred in the SAP group at 30 minutes, whereas significant postope-rative MAP reductions occurred in the PECS group at 6 and 8 hours.
DISCUSSION
Modified radical mastectomy is related to a higher incidence of acute and chronic pain as well as PONV, which was the primary motivation for conducting this study. The study aimed to evaluate the analgesic quality in PECS II block compared to SAP block for the treatment of postoperative pain after MRM.
Our results showed that patients undergoing MRM who received SAP block had a significantly longer time to rescue analgesia compared to those who received PECS II block. In addition, SAP block significantly lowered the total need for opioid, the patients’ need for analgesia, and VAS scores immediately at PACU and at 2, 8, 20, 22, and 24 hours postoperatively. Meanwhile, compared to the PECS II, the SAP procedure needed longer time to perform the block. These differences in opiate intake, VAS ratings, and block execution time were statistically significant but not clinically significant. However, PECS II and SAP blocks had comparable intraoperative fentanyl consumption, surgeons’ satis-faction, and PONV.
Comparable with previous studies [25, 26], ASA status, presence of chronic diseases, and duration of surgery showed no significant differences between the SAP and PECS II groups. Meanwhile, the current study revealed significant differences regarding age and body mass index between the groups. This could be attributed to the small number of our patients, as small trials might be prone to baseline imbalance [27]. In addition, age and body mass index were not significantly correlated with VAS score at PACU and 24 hours; thus age and body mass index were not correlated with the outcomes.
In addition, our results were in agreement with Ali et al. [28], who noted that SAP block can provide greater pain relief for the first 6 hours postoperatively than thoracic epidural analgesia among breast surgery patients. Chong et al. [29] conducted a systematic review and meta-analysis of randomised, controlled trials comparing adult patients who underwent SAP block, non-block, and paravertebral block for postoperative analgesia. Compared to non-block, the SAP technique prolonged the time to first analgesic requirement, reduced the postoperative pain scores and opioid need and prevented nausea and vomiting. However, SAP block was not significantly different from paravertebral block. In PECS II block, the anaesthetic medication leaks and ultimately reaches the target, while in serratus block, the anaesthetic agent is injected directly above the outflow of the lateral cutaneous nerve through the midaxillary line. Therefore, Blanco et al. [15] concluded that the serratus block produced better axillary analgesia than PECS II. Wang et al. [14] supposed that SAP could paralyse a large number of intercostal nerves, which may innervate the mammary gland and the lateral and anterior cutaneous branches of the second to sixth thoracic intercostal nerves [30, 31].
However, Bakeer et al. [25] compared SAP, PECS II, and no blockade in 180 women following MRM. They found that the PECS-II and SAP groups had considerably longer time to first rescue analgesia, reduced morphine requirements in the first 24 hours, lower intraoperative fentanyl use, and lower VAS scores. Meanwhile, both PECS II and SAP blocks had similarly acceptable analgesia. Kubodera et al. [32] reported no differences in postoperative pain or opioid use within the first 24 hours in 43 women undergoing mastectomy with either PECS II or SAP blocks; however, this was a small retrospective study.
A randomised, controlled trial [33] found no difference in acute postoperative pain scores between SAP and PECS II blocks in patients undergoing mastectomy. Compared to no block, patients who received SAP or PECS II had an equivalent decrease in static and dynamic postoperative pain levels [26]. However, the mean difference in pain scores on the numeric rating scale was less than 2; therefore, it was unlikely to be clinically significant. Abdallah et al. [34] observed in their retrospective cohort study that PECS I had the same analgesic effect as the serratus block, where both PECS I and serratus blocks had comparable reductions in intraoperative fentanyl demand, postoperative morphine intake, pain scores, and PONV.
Several studies [20, 21] support the claim that the PECS II block is more efficient than the SAP block based on indirect comparisons with paravertebral blockade. Patients receiving PECS II block in addition to general anaesthesia had reduced VAS scores and postoperative morphine doses, according to Bashandy and Abbas [35]. Wang et al. [36] reported significant reduction in postoperative morphine and intraoperative fentanyl consumption, less PONV, and lower VAS scores in pectoral nerve block patients compared with general anaesthetic patients.
The inconsistency of the present results with those of previous studies could be attributed to the higher volume of local anaesthetics (15–20 mL) injected between the pectoralis major and minor in those studies compared to ours (10 mL). This enormous volume may have extended to the axilla, obstructing the thoracic intercostal nerves. Furthermore, the volume of local anaesthetics injected in the serratus block (20–25 mL) was less than that used in our research (30 mL). Variations in the type of operation may cause some discrepancy. All our patients underwent MRM, while lumpectomy coupled with dissection of the sentinel node and a small percentage of MRM were done in other studies.
In this study, the haemodynamic parameters were comparable in both the SAP and PECS II blocks during most of the intraoperative and postoperative time. This agrees with Blanco et al. [15], who performed the SAP block in 50 females who were scheduled for modified radical mastectomy and found that the SAP block had no effect on haemodynamics because no sympathetic block was present. However, Kaur et al. [26] reported better control of haemodynamics in PECS II block than in serratus inferior block. The PECS II block can cover the anterior cutaneous branches of the intercostal nerves directly and more quickly than the serratus inferior block. Fajardo et al. [37] reported that the pectoral nerve block regulated the haemodynamic parameters better than the serratus block, which was attributed to the use of dexamethasone before the block, which may have delayed the beginning of block action [38]. This inconsistency of results might be attributed to differences in the serratus block techniques, where we did not administer dexamethasone before the block, and we used the serratus anterior block instead of the serratus inferior block.
Few patients in our study experienced PONV, which is consistent with the findings of earlier research work [39, 40]. This was explained by the study’s low opioid usage and may be as a result of the use of prophylactic antiemetics prior to surgery. Generally, the PECS and SAP blocks are considered safe procedures. However, a few potential consequences might occur including inadvertent intravascular injection, pneumothorax, hematoma at the injection site, and block failure [41]. For better observation of the structures and prevention of local anaesthetic spread, we performed the block using echogenic needles and guided by US.
We estimated the range of the block and checked for consequences such as pneumothorax by studying the spread of local anaesthetics on US. In addition, the use of US-guided PECS II or SAP blocks requires considerably more time and effort from anaesthetists than blind techniques. We found that the total time of SAP block was longer than that of PECS II block, with no statistically significant difference between surgeons’ satisfaction in both blocks.
The use of a uniform amount and concentration of local anaesthesia in both blocks is one of our study’s strengths (30 mL of 0.25% bupivacaine). As a result, when compared to prior studies that used unequal volumes and/or concentrations of local anaesthetic, our methodology provided a fair analgesic comparison between groups.
We could not assess dermatome distribution before surgery because we conducted the block procedures after anaesthetic induction when the patient was unconscious. Non-blinding of the anaesthetist performing the block, the small sample size and being a single-centre study were other limitations. However, the person collecting the data was unaware of the group distribution.