Purpose
Malignancy of corpus uteri accounts for 4.8% of all malignancies in women, making it the second-most common gynecological malignancy worldwide [1]. In India, endometrial carcinoma accounted for 16,413 new cases and 6,385 deaths in 2020 [2]. The standard treatment for endometrial cancer is total abdominal hysterectomy, with bilateral salphingo-oophorectomy and surgical staging. Adjuvant brachytherapy with or without external beam radiation therapy (EBRT) are integral components of adjuvant therapy in selected patients [3]. There are many different applicators, dose-fractionation schedules, and treatment planning techniques, which all result in favorable clinical outcomes and low rates of toxicity. Recommendations have been published by the American Brachytherapy Society (ABS) and the American Society of Therapeutic Radiation Oncology (ASTRO) to help guide practitioners in the use of vaginal brachytherapy [4, 5].
In majority of centers, endometrial carcinoma brachytherapy is delivered to vaginal vault using either intra-vaginal ovoids or cylinders. Whether cylinder and ovoid can be used inter-changeably for vault brachytherapy, is a question that remains unaddressed in the ABS recommendations. In the era of image-guided brachytherapy, volume-based brachytherapy for vaginal vault needs to be evaluated. In the present study, we intended to compare the dose volume parameters achieved using ovoids vs. vaginal cylinder for vault brachytherapy with image-based planning. The aim of the study was the comparison of dose volume parameters of the target and organs at risk in vaginal vault brachytherapy using ovoids versus cylinder in endometrium carcinoma.
Material and methods
Study design and study population
The study was a prospective, single-institute, observational, two-arm, comparative study, and it was carried out in the Department of Radiation Oncology at a tertiary cancer center after obtaining clearance from the institutional scientific review board and ethics committee. The study included 25 histologically proven post-operative cases of endometrial carcinoma requiring brachytherapy, with satisfying inclusion and exclusion criteria. Patients with Eastern Cooperative Oncology Group (ECOG) 0-2 suitable for intra-vaginal brachytherapy, who consented to participate in the study were included. Patients with clear cell serous carcinoma histology, and those who were suitable for interstitial brachytherapy were excluded from the study.
Pre-brachytherapy evaluation
Written informed consent was obtained from all patients. A complete clinical evaluation was carried out, and details of symptoms and their duration as well as treatment received, such as surgery and external radiotherapy, were acquired. Blood investigations, including complete blood count, liver and renal function tests, COVID-19 test, and serology (HBsAg, HIV, and HCV) were performed before the procedure. Per vaginal examination was done to know whether the vault was healed, to assess the size of cylinder and vaginal ovoids, and to evaluate for any residual and recurrent disease in the vagina and parametrium. Laxative was provided for two days prior to the procedure to ensure the rectum was empty, according to institutional pre-treatment protocol.
Procedure
In brachytherapy minor operation theatre, with aseptic precautions, the patient was put in a lithotomy position, parts painted and draped. Per vaginal examination was performed to confirm that the vault was healed, and to choose the proper size of applicator. The urinary bladder was catheterized using Foley’s catheter, and the bulb was inflated with 7 cc distilled water.
Ovoids application: Manchester’s ovoids were used in all patients, and the largest ovoids that could fit were selected. Semi small ovoids (diameter, 2 cm) were applied in 4 patients, small ovoids (diameter, 2.5 cm) in 10 patients, and medium-sized ovoids (diameter, 3 cm) in 11 patients. Care was taken to ensure that medial ends touched each other to avoid any underdosing of the vault. Adequate anterior and posterior packing was done with betadine and lignocaine-soaked gauge. The applicator position was secured, and the patient was sent for simulation.
Cylinder application: Single-channel vaginal cylinder was applied in all patients. The length and diameter of the vagina were recorded during per vaginal examination, and the largest cylinder that could fit was selected. Diameters of the cylinder ranged from 2.5 and 3.5 cm. 2.5 cm diameter cylinder was used in 5 patients, 3 cm diameter cylinder in 16 patients, and 3.5 cm diameter cylinder in 4 patients. The cylinder was covered with a condom. Lignocaine jelly was applied around the applicator, and the cylinder was inserted into the vagina. The applicator position was secured, and the patient was sent for simulation.
Computed tomography (CT) simulation: The patient was simulated with Philips large bore CT (Philips Medical System, Cleveland OH, USA) simulator scanner. The bladder was filled with 30 ml of saline with 1 ml of contrast. A rectal tube was inserted and deflated. Slices of 3 mm thickness from L3 to 5 cm below ischium were taken, and images were pushed to Eclipse treatment planning system (Varian Eclipse Brachy Vision, version 15.6).
Contouring and planning procedure: Organs at risk (OARs), such as the bladder, rectum, and urethra were contoured on CT slices according to the Groupe European Curietherapie, European Society of Therapeutic Radiation Oncology (GEC-ESTRO) guidelines [6]. The vault and upper half (3-4 cm) of the vagina was contoured as clinical target volume (CTV), and the vault was also contoured separately from CTV.
Published guidelines are not available for contouring of the vagina as an organ. In a study by Susko et al., the vagina was contoured as a three-dimensional organ on CT images. In their study, they used a 0.4 cm fixed brush to outline the applicator and packing, and expanded to include any additional vagina visualized [7]. We used the same protocol to contour upper 3-4 cm of the vagina. The applicators were then digitally reconstructed, with dwell position and dwell times specified. For vaginal cylinders, prescription points were defined 5 mm from the surface of the cylinder, and for ovoids, prescription points were defined 5 mm laterally from their surface. Planning was done using Varian Eclipse BrachyVision, version 15.6. The dose prescription was 6-7 Gy at 0.5 cm from the surface of the applicator. The upper 3-4 cm of the vagina was taken as treatment length. Normalization was done, so that 100% dose was achieved to prescription points. As per the institutional protocol, optimization was done to ensure that 100% dose does not fall on the mucosa of normal organs, which consistently happens when a dose is prescribed at 5 mm from the applicator surface. This was achieved using graphical optimization, where isodose lines were dragged manually to cover CTV or dragged away from OARs as required, hence, changing dwell times and positions. The same optimization method was applied for the cylinder and ovoids. Each plan was evaluated both quantitatively and qualitatively using dose-volume histogram, and by visualizing dose distribution in each slice of CT. Contouring procedure, planning, and optimization were carried out by the same clinician and physicist to obtain the maximum possible reproducibility and to minimize inter-observer bias. The optimal plan was approved and sent to afterloader for treatment delivery. Patients received vaginal brachytherapy with cylinder and ovoids application alternatively on weekly basis for comparison.
HDR source
Patients were treated with Varian GammaMedplus iX HDR brachytherapy afterloader with iridium-192 stepping source. 0.5 cm source position spacing was applied [8]. Dose calculations were based on TG-43 formalism [9].
Data collection and analysis
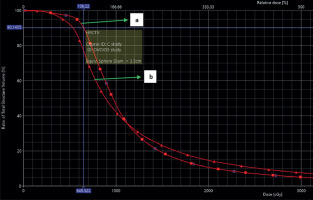
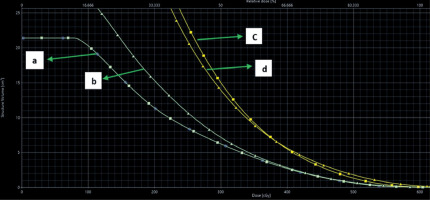
Dose-volume histogram of OARs and CTV was generated. Dose parameters were recorded according to GEC-ESTRO recommendations. D0.1cc, D1cc, D2cc, and mean doses were recorded for the bladder, rectum, and urethra. Mean, D100, D90, D50, V150, V100, V90, and V50 were documented for CTV volume and vault separated from CTV.
D50, D90, and D100 were defined as the dose received by 50%, 90%, and 100% of clinical target volume, respectively. V50, V90, V100, and V150 were defined as the percentage of clinical target volume receiving 50%, 90%, 100%, and 150% of prescribed dose, respectively. D0.1cc, D1cc, and D2cc were the minimum doses to the highest irradiated 0.1 cc, 1 cc, and 2 cc areas of OARs, respectively.
Statistical analysis
All study data were manually entered into an Excel spreadsheet. Data were analyzed using SPSS version 23 (IBM software) for Windows. Dosimetric parameters were expressed as mean and standard deviation. Paired t-test was applied to compare dose volume parameters between ovoids and cylinder plans for every patient. Statistical tests were done with SPSS version 23, and p-value < 0.05 was considered statistically significant. Normality of sample differences was assessed using Q-Q plots and Shapiro-Wilk test. Both methods indicated that differences between paired observations did not significantly deviate from normal distribution (p > 0.05).
Results
Patient and tumor characteristics are presented in Table 1. All the patients underwent both cylinder and ovoids application alternatively on weekly basis. The third fraction was decided based on dosimetric superiority of the application for particular patient. Re-simulation can be done if vaginal ovoids are used with varying vaginal packing. However, in the current study, the third fraction without a new simulation was executed. The length of the vagina treated was upper 3-4 cm. The prescription dose for vaginal brachytherapy was 6-7 Gy to 0.5 cm from the surface of the applicator. Details of brachytherapy treatment are described in Table 2.
Table 1
Patient and tumor characteristics
| Mean age (years) | 55.6 ±8.2 | |
| Stage and number of patients | IA | 1 patient |
| IB | 6 patients | |
| II | 4 patients | |
| III | 14 patients | |
| Histologic type and number of patients | Endometrial carcinoma | 25 patients |
Table 2
Details of brachytherapy treatment
Dosimetry
Dosimetric comparison of CTV (vault and vagina) and vault (separated from CTV) are shown in Tables 3 and 4, whereas dosimetric results of the bladder, rectum, and urethra are provided in Table 5.
Table 3
Comparison of target (CTV) doses between cylinders and ovoids
Table 4
Comparison of vault doses between cylinders and ovoids
Table 5
Comparison of doses to organ at risk between cylinders and ovoids
Comparison of target (CTV) and vault doses between cylinders and ovoids
The mean values of D90, D50, V150, V100, V90, and V50 of CTV were comparable between the cylinder and ovoids plans. The mean dose of CTV was significantly higher with cylinder plans than ovoids plans, and D100 of CTV was significantly higher with ovoids plans than cylinder plans (mean = 15.63 Gy vs. 14.64 Gy, p = 0.016, and D100 = 37.82% vs. 42.86%, p = 0.042, for cylinder vs. ovoids; Table 3).
Similarly, when we analyzed the dosimetry of the vault alone, D90, D50, V100, V90, V50, and mean of the vault did not show any statistically significant difference between cylinder and ovoids. The V150 of the vault was significantly higher with cylinder plans than ovoids plans, and D100 of the vault was significantly higher with ovoids plans than cylinder plans (V150 = 14.81% vs. 6.86%, p = 0.02, and D100 = 37.77% vs. 44.80%, p = 0.029, for cylinder vs. ovoids; Table 4).
Examples of dose distribution in cylinder and ovoids are shown in Figures 1 and 2. Dose volume histogram (DVH) of CTV and organs at risk are represented in Figures 3 and 4.
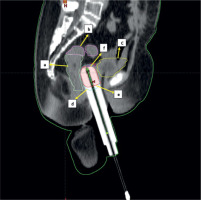
Fig. 1
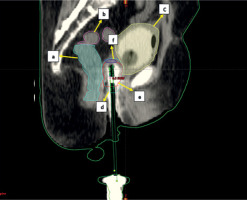
Sagittal section of CT scan of cylinder application with isodose distribution: a – rectum (light green), b – sigmoid (magenta), c – bladder (yellow), d – 100% isodose level (cyan), e – CTV (red), f – vault (navy blue)
Comparison of doses to organs at risk between cylinders and ovoids
The D0.1cc, D1cc, D2cc, and mean for the bladder, rectum, and urethra were comparable between the cylinder and ovoid plans (Table 5).
Discussion
Vaginal vault is equivalent to level 1 section of the vagina. It is measured posteriorly from posterior vaginal wall (apex or highest part of the vagina) to 2.5 cm below this point. It comprises the anterior, posterior, and two lateral fornices [10]. Vaginal vault brachytherapy is used in post-operative and local recurrent cases of endometrial carcinoma as brachytherapy treatment alone or in combination with EBRT. The ABS states that the choice of applicator is both institutional- and patient-dependent. Ovoids treat only the upper part of the vagina, whereas a vaginal cylinder allows treatment of the entire length of the organ. The shape of the vagina also determines the applicator selection. Cylindrical-shaped vagina is adequately treated with vaginal cylinder. In some patients, the lateral fornices are expanded because of surgical remnant of vaginal fornices (dog-ear configuration). They are treated better with ovoids. The ABS recommends that the proximal 3-5 cm of the vagina should be treated, and for clear cell and serous histology, the entire length of the vaginal canal should be treated. The ABS also recommends that the prescribed dose is to be delivered either at the vaginal surface or at 0.5 cm depth depending on an institutional policy. Moreover, the ABS suggests reporting doses at the vaginal surface and 0.5 cm mucosal depth regardless of prescription point [3, 5]. In the literature, very few studies compare the dosimetry between the cylinder and ovoid applications [11-13]. Studies evaluated two different sets of patients receiving ovoids and cylinder application [11]. To the best of our knowledge, no direct comparison exists between these two applicators in the same patient. Here, 25 endometrial carcinoma patients, who received both cylinder and ovoids application on weekly basis were compared.
Kumar et al. [11] evaluated 15 endometrial carcinoma patients, each of whom received vaginal brachytherapy with ovoids and cylinder applicators. In their study, the upper 2 cm of the vagina was contoured as the target volume. The dose prescribed was 21 Gy in 3 weekly fractions at 0.5 cm from the surface of applicator. They analyzed point doses to the bladder and rectum, and D100 and V100 of the target volume. The mean dose to the rectum and bladder was significantly better with ovoids. V100 and D100 results were significantly better with cylinders compared with ovoids. The authors demonstrated that the use of a vaginal cylinder resulted in a higher dose to OAR, and a more homogenous dose to the vault. Also, the treatment with ovoids resulted in a less homogenous dose to the vault with a lower dose to OAR [11]. In our study, we observed that doses to normal organs at risk (bladder, rectum, and urethra) were comparable between the cylinder and ovoid plans. Contrary to Kumar et al. work, in our study, D100 was high with ovoids (p = 0.042), and V100 was comparable between the two applicators. We observed slightly increased, but not statistically significant, CTV coverage with cylinder compared with ovoids in terms of D90 (94.63 vs. 91.06), V90 (92.59 vs. 90.21), and V100 (88.01 vs. 85.62). However, these differences were not statistically significant. D100 of CTV was significantly less with cylinder plans (37.82 vs. 42.86, p = 0.042), and there was a significant increase in mean dose (15.63 Gy vs. 14.64 Gy, p = 0.016) in cylinders compared with ovoids. The increase in mean dose of cylinder plans may be due to the excess of vaginal packing in some ovoids cases, which may underdose the vaginal wall and mean dose in ovoids.
Zingoni et al. [12] discussed the influence of applicator shape in vaginal cuff brachytherapy. Their study showed that average doses to OARs were lower using ovoids compared with cylinders. On the contrary, our study did not show any significant difference in OARs doses. Chapman et al. [14] evaluated underdosing of vaginal cuff in cylinder brachytherapy using magnetic resonance (MR) simulation images. Thirty-two endometrial cancer patients underwent MR simulation after application insertion. The vaginal cuff was contoured up to 4 cm from the tip of applicator. The authors reported that in two-thirds of patients, vaginal cuff was underdosed. They also observed that over 69% (i.e., two-thirds) of patients had at least 1 cc of vaginal cuff that received less than 75% of prescription dose, and 50% of patients had at least 1 cc of vaginal cuff that received less than half (50%) of prescription dose. The mean minimum point dose to vaginal cuff was 2.4 Gy, or 34% of intended prescription dose [14]. In our study, vault coverage was analyzed with parameters, including D100, D90, D50, V100, V90, V50, V150, and mean dose. The D90, D50, V100, V90, V50, and mean of the vault did not show any statistically significant difference between cylinders and ovoids. The V150 was significantly higher with cylinder plans than ovoids, and D100 of the vault was significantly higher with ovoids plan. We used a CT scan for simulation, and maximum 2 cm was contoured for the vault from the tip of the applicator. Single-channel cylinder would underdose vault if the thickness is beyond 0.5 cm. In order to avoid 100% of dose reaching organs at risk, we drag the 100% isodose lines away from OARs, hence decreasing D100 of cylinder compared with ovoids, which had two source channels. Cylinder with single central channel may limit the adjustment of dose distribution. Single-channel may not always provide optimal target dose, while keeping the critical structure dose below tolerance limit. Multichannel vaginal cylinder, i.e., Miami applicator, overcome these limitations, and can be used for small tumors extending up to 1 cm depth. Multichannel cylinders provide asymmetric dose distribution, such that the vaginal mucosal dose is kept low in all areas (except for small areas where tumor is present), and also normal organ doses may be reduced [15].
In a study by Kim et al., dosimetric comparisons of ovoids and cylinders were performed, and the potential of underdosing vaginal surface because of colpostat separation and vaginal packing was investigated. The separation of ovoids and vaginal packing considerably reduced the vaginal surface dose at the apex and near the rectum and bladder, and led to cold spots. Furthermore, the amount and location of packing can be very inconsistent during each application in clinical practice. Because our goal is to treat the vaginal mucosa, the largest possible diameter of colpostat should be used rather than vaginal packing around the colpostat, or colpostat separation. Although the clinical implication of these dose reductions is unclear, the potential for local recurrence remains a concern [13]. Gap between the medial ends of ovoids will cause a dip in isodose curve and underdose the vault. Therefore, care should be taken to ensure the medial ends are close to each other and not to separate ovoids. If there is a gap between 2 ovoids, larger ovoids should be considered. Gap between two ovoids should be kept to a minimum, and the packing of vagina should be done with great care to avoid generating cold spots along the upper vaginal surface. In our study, CTV mean dose was significantly low in ovoids plans, which may be due to an excess of vaginal packing used in a few cases that compromised the dose received by the posterior vaginal wall in the last few slices of CTV. The difference in dose distribution with a gap and no gap between ovoids are illustrated in Figures 5 and 6. Larger sample size of patients and multicentric comparisons between ovoids and cylinders will be beneficial for recommendations and guidelines.
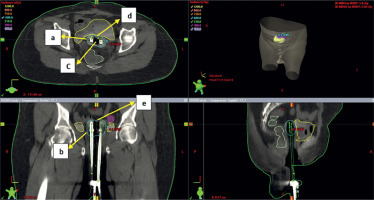
Fig. 5
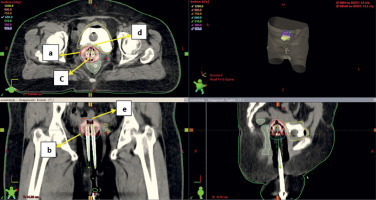
Axial section (upper left), coronal section (lower left) and sagittal section (lower right) of CT scan of ovoids application with more gap between medial ends of ovoids. a, b – 100% isodose level (cyan), c – gap between medial ends of ovoids, d, e – dip in the 100% isodose line. The gap between medial ends of ovoids will cause dip in isodose curve and under dose the vault
Conclusions
To the best of our knowledge, no direct comparison exists between cylinder and ovoids in terms of target coverage and dose to OAR. The present study showed that the dose to normal OAR was comparable between the cylinder and ovoid plans. Most of dosimetric parameters of CTV and vault were comparable between the cylinder and ovoid plans. Both applicators provide good reproducibility. Excessive packing should be avoided in ovoid application, as it may compromise the dose received by vaginal walls. The choice of applicator will ultimately depend on the institutional policies and oncologist decision. However, in patients with dog-ear configuration of the vagina, ovoids may be the preferred choice according to the ABS guidelines.