Purpose
For cervical brachytherapy planning, magnetic resonance imaging (MRI) is preferable to computed tomography (CT) for target delineation [1]. The advantages to MRI-guided brachytherapy are numerous, including excellent soft tissue contrast between residual tumor, cervix, and organs at risk (OARs) [2]. However, due to logistical and financial restrictions, in-room MRI is sometimes not routinely available in brachytherapy centers [3]. Our institution has created a workflow that integrates MRI-based target delineation with an in-room CT scanner, with the aim of improving target coverage and conformality [4]. This study reports the initial dosimetric results with using this workflow.
Material and methods
Institutional review board approval was obtained for the entirety of this study.
Technique
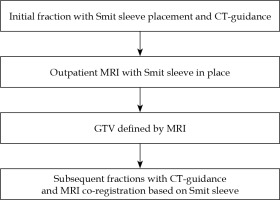
Our method has been previously described and summarized in Figure 1 [4]. In summary, as of 2014, we performed an outpatient MRI after the first fraction of brachytherapy for primarily two reasons: 1. To assess response to chemoradiation, and 2. Target volume assessment for brachytherapy planning. The first fraction is entirely CT-based, under general anesthesia, and a Smit sleeve is placed. Prior to subsequent brachytherapy fractions, an outpatient MRI (multiplanar multisequence, T2-weighted) is performed. For each subsequent tandem and ovoid insertion, the MRI was rigidly co-registered to the CT based on the Smit sleeve. The high-risk clinical target volume (HR-CTV) is defined as the entire cervix, cervix tumor as defined by the CT for the first fraction, and MRI registration for fractions 2-5. Organs at risk are contoured on the CT dataset for each fraction and include bladder, rectum, and sigmoid for all patients. No deformable registration techniques were performed.
Fig. 1
Institutional workflow. The Smit sleeve is placed under anesthesia. The MRI between the first and subse- quent fractions is performed outpatient, separate from brachytherapy procedure, and is also used for external beam response assessment
CT – computed tomography, GTV – gross tumor volume, MRI – magnetic resonance imaging
Analysis
In this analysis, dosimetry from the first fraction is compared to the average of subsequent fractions using a matched, two-tailed, paired t-test. Average, minimum, maximum, and standard deviation of dosimetry changes were calculated. Patients were excluded if they did not receive concurrent sensitizing cisplatin or if a modified fractionation regimen (e.g. 3-4 total brachytherapy fractions) was utilized, as their tumor response may differ. The SPSS Statistics software package (version 24, IBM Corporation, Armonk, New York, NY, USA) was used for all analyses.
Results
Patient, disease, and treatment characteristics
Forty-six consecutive patients, treated between September, 2014 and June, 2017, were identified who received definitive chemoradiation with 5 fraction intracavitary HDR brachytherapy for cervical cancer with MRI fusion utilized for fractions 2-5. Median age at time of brachytherapy was 49.9 years old (range, 31.6-71.4 years old). The majority were squamous cell histology (82.6%), FIGO stage (2009) IB2 or IIB (25.5% and 39.2%, respectively), and staged with an MRI (60.9%) and positron emission tomography (PET)/CT (89.1%). Table 1 presents patient, disease, and treatment characteristics.
Table 1
Patient, disease, and treatment characteristics
[i] CTV – clinical target volume, D2cc – maximum dose received by 2cc of the volume, D90% – maximum dose received by 90% of the volume, EBRT – external beam radiation therapy, ECOG – Eastern Cooperative Oncology Group performance status, EQD2 – equivalent total dose in 2-Gy fractions (assuming α/β of 10 Gy for tumor and 3 Gy for normal tissues), FIGO – International Federation of Gynecology and Obstetrics, MRI – magnetic resonance imaging, PET – positron emission tomography
Dosimetry with or without MRI
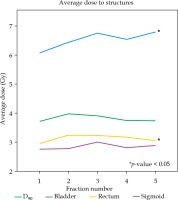
The D90 to the HR-CTV was increased from fraction 1 to fraction 2-5 averaged for individual patients using a match pair analysis (6.07 Gy vs. 6.64 Gy, respectively; p = 0.017). The range in change of D90 was –5.41 to 3.75 Gy, with 31/46 (67.4%) experiencing an increase. The D2cc to rectum was also increased from fraction 1 to fraction 2-5 averaged (2.96 Gy vs. 3.17 Gy, respectively; p = 0.01). The range in change of D2cc to rectum was –1.28 to 1.63 Gy, with 32/46 (69.6%) experiencing an increase. There was no significant difference in dose to the bladder or sigmoid with respect to MRI registration for the individual patient, as shown in Table 2. Figure 2 represents the average dose to structures by fraction.
Table 2
Matched-pair analysis comparing dosimetric values from CT only based planning to MRI-based planning
| Fraction 1 (Gy) (CT-guided) | Fraction 2-5 average (Gy) (MRI-fused) | P-value | |
|---|---|---|---|
| D90% HR-CTV | 6.07 | 6.64 | 0.017 |
| D2cc rectum | 2.96 | 3.17 | 0.01 |
| D2cc bladder | 3.72 | 3.84 | 0.442 |
| D2cc sigmoid | 2.76 | 2.87 | 0.29 |
Fig. 2
Mean dose to structures by fraction number for the entire cohort (n = 46). Fractions 2-5 were based on MRI for HR-CTV delineation. D90, maximum dose received by 90% of the volume to the HR-CTV
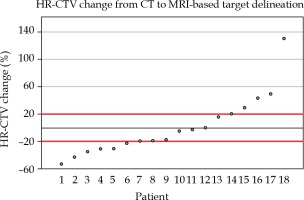
In order to quantify the impact on HR-CTV volumes, we measured the percent change from CT to MRI-based target delineation for the eighteen patients, with complete volumetric data. As shown in Figure 3, 11/18 (61.1%) were found to have a greater than 20% change in HR-CTV (6 decreased and 5 increased). The mean absolute HR-CTV change was 7.37 cc (standard deviation, 7.25 cc), and the range of volume changes in HR-CTV was –53.09% to 130.05%.
Discussion
While no change in overall target volume was found, the current study suggests that physicians are making individualized target changes to prevent either over- or under-coverage, based on the MRI fusion. MRI fusion resulted in higher dose to the HR-CTV across patients, although at the cost of higher rectal doses, which were still within recommended constraints. The higher rectal doses observed may be the result of more aggressive HR-CTV coverage mandated by the MRI-defined gross residual disease. Importantly, this approach is applicable to centers without MRI availability within the department.
Brachytherapy is a critical component of definitive therapy for cervical cancer and should be made available to all patients [5]. GEC-ESTRO promotes adaptive brachytherapy, using a volume-based approach for each fraction [6]. MRI for each fraction with applicators in place is ideal; however, MRI availability is not always readily accessible at most centers. An alternative is using MRI for the first fraction and CT for subsequent fractions. Viswanathan et al. have shown CT to have significant differences in D90 and volume treated with prescription dose compared to MRI-based planning [7]. Our method is particularly attractive because it allows centers to obtain an MRI on an outpatient basis that can by itself result in meaningful volume adjustments in the majority of patients. This study is particularly interesting that the HR-CTV volume can both be over- and under-estimated with CT.
A prospective multicenter study from France reported an improved local control and toxicity with the transition from 2D to 3D planning [8]. Long-term outcomes from prospective studies with MRI-based planning are maturing and promising [9]. Clinical outcomes using our described technique have been previously reported, although is limited by retrospective data and patient heterogeneities [10]. When compared to CT only brachytherapy, the MRI fusion method resulted in fewer late toxicities and equivalent local control. The current study is unique because we evaluated patient-specific volume changes between fractions, as opposed to comparing treatment with or without MRI. Better tumor visualization allows for manual dose optimization techniques theoretically improving therapeutic ratio for each patient [11]. Further optimization of imaging protocols in interstitial setting for locally advanced tumors are worth investigating in future studies.
Our technique and present analysis have limitations. First, MRI is only performed once, and therefore we would not capture ongoing changes during brachytherapy treatment. Second, while a Smit sleeve is in place during MRI and assists with co-registration, tissue deformity by applicators must be accounted for on CT by the treating physician for each fraction. Limitations to this analysis include a small number of patients with full volumetric data, although HR-CTV volume changes appeared as normally distributed in both directions, implying these expected changes with MRI fusion can be applied to a larger cohort. Lastly, a large number of patients with long-term follow-up using this technique is required to validate clinical superiority to CT only based planning.
Conclusions
The use of asynchronous MRI for target delineation, with co-registration to CT for each fraction of brachytherapy was associated with higher D90 to the HR-CTV. We observed slightly higher D2cc rectal doses with MRI fusion, but cumulative rectal doses were within accepted thresholds. High-risk target volumes were not consistently increased or decreased, but MRI fusion was associated with target volume changes greater than 20% in over half of the treated patients.