Sepsis is the most frequent cause of mortality in patients with acute illness worldwide [1]. Delays in the identification of sepsis and its management often result in rapid deterioration to circulatory collapse, multiple organ failure, and eventually death [2]. Therefore, prompt diagnosis of sepsis and rapid initiation of treatment can positively impact patient outcomes and reduce costs [3, 4]. Sepsis is defined as a dysregulated immune response to an infectious insult, which results in life-threatening organ dysfunction [5].
A positive microbiological culture is an accepted benchmark for distinguishing sepsis from non-infectious conditions. However, bacteria may take a long time to grow, and during this phase, the condition of the patients may promptly decline. To date, no single ideal biomarker for sepsis has been identified [6]. Thus, there is an urgent need for a biomarker that can identify sepsis in an early stage as well as help in assessing the prognosis such that an appropriate antimicrobial agent or a combination thereof may be initiated on time [7].
Currently, several biomarkers such as serum lactate, serum procalcitonin (PCT), and quantitative C-reactive protein (QCRP) are being used. PCT level alone is inadequate to diagnose invasive bacterial infection and assess its severity, as it has been observed to rise in several non-septic conditions as well. Neutrophil CD64 (nCD64) is a high-affinity immunoglobulin Fcγ receptor that is constitutively expressed on monocytes and eosinophils. Recently, a number of studies have investigated the role of nCD64 expression in the diagnosis of bacterial infection and sepsis [8]. Many studies have revealed that nCD64 is expressed only at low levels by neutrophils in healthy hosts under non-septic conditions, and fewer than 2000 CD64 molecules are found on normal neutrophils. Therefore, nCD64 expression may be used as a biomarker for detecting systemic infection and sepsis in various populations such as adults, children, and neonates [9–11]. In patients with sepsis, reduced monocytic cell surface expression of another biomarker, monocytic HLA-DR (mHLA-DR), has been detected in circulating monocytes; there is now a consensus that decreased mHLA-DR expression is a consistent marker for the development of immunosuppression in critically ill patients [12, 13].
Flow cytometry (FCM) has been demonstrated as a useful diagnostic tool for the identification of immune-related disorders for decades. It is a technique for profiling and organising cells or other particles by illuminating them when they flow in front of a light source. Variations in circulating cytokines and surface markers (e.g., neutrophil CD64 and mHLA-DR) may help in understanding the mechanism of the systemic response to infection and discovering new diagnostic/prognostic assays.
The present study was conducted with the aim of measuring the expression of serum PCT, QCRP, nCD64, and mHLA-DR in patients admitted to the ICU and to correlate the expression of these biomarkers to predict the progression and outcomes of sepsis.
METHODS
This study was approved by the Institutional Review Board (IEC No. 78/17) and written informed consent was obtained from the legal guardian of all subjects participating in the trial. The trial was registered prior to patient enrolment in the Clinical Trial Registry of India (www.ctri.nic.in) (CTRI/2019/07/027286, date of registration: 31st July 2019). This study was a tertiary centre based longitudinal cohort study.
Patients who fulfilled the following inclusion and exclusion criteria were enrolled in the study:
Inclusion criteria:
age of the patient > 18 years,
patient developing sepsis during an observation period of 20 days in the intensive care unit (ICU),
sequential organ failure assessment (SOFA) sco-re ≥ 2,
patient with sepsis at the time of admission to the ICU,
patient with culture-positive source of infection.
Exclusion criteria:
patient’s or relative’s refusal to give consent,
age of the patient < 18 years,
patient who developed sepsis after 20-day observational period in the ICU,
patient who developed sepsis after recovering from 1st episode of sepsis,
patient on granulocyte colony stimulating factor therapy,
patient on immunosuppression therapy,
patient with negative culture test.
The participants were included from the ICU of a tertiary care hospital in Northern India. Data collection for the study spanned the period from 1st August to 30th November 2019. Targeting a sensitivity of 80% of novel parameters with an observed sepsis prevalence of 28% in adult patients admitted to the ICU, the sample size was calculated using the formula:
{Z2 × Sensitivity(1 – Sensitivity)/(margin of error)2}/Prevalence
where Z = confidence interval (95%), sensitivity = 80%, margin of error = 20%, prevalence of sepsis in adults among ICU patients = 28%.
{(1.96)2 × 0.8 (1 – 0.8)/(0.2)2}/0.28 = 54.8
(rounding up to 55)
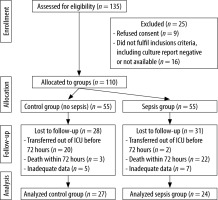
We enrolled a total of 110 patients (55 patients with sepsis (cases) and 55 patients without sepsis (controls)) and each patient was followed until their final outcome (death or discharge). Due to various factors such as death within 24 hours, sterile culture, and inadequate data, we were able to analyse data from 27 cases and 24 disease controls. Subjects enrolled in the study were those in whom sepsis was suspected according to history, clinical examination, and Survival Sepsis Campaign Guidelines for Sepsis-3 and confirmed by relevant culture. Routine laboratory investigations including serum procalcitonin and quantitative CRP were carried out and disease severity (severity of organ dysfunction) was assessed using the Sequential Organ Failure Assessment (SOFA) score. Subjects were divided into two groups (sepsis and disease controls/non-sepsis) based on the clinical diagnosis, which was supported by laboratory investigations. Disease severity in the ICU was assessed and documented using the SOFA score.
Blood samples for routine laboratory investigations such as haemoglobin level, total leukocyte count (TLC), platelet count, blood culture, and other body fluid culture measurements were collected at the time of admission or on arrival at the ICU and before delivering the first dose of antibiotic(s) at time D0/D1. The study biomarkers were estimated at the same time as that of TLC, i.e., within 24 hours of admission (D0/D1) and on the third day from admission to the ICU (D3). The SOFA scores were also determined for D1 and D3. Blood for bacterial culture was obtained at the time of admission (T0) before administering the first dose of antibiotic(s).
Assay procedures
A minimum of 10 mL plus 10 mL of paired venous blood sample was placed in the Becton Dickinson (BD) adult culture broth bottles after the site for venepuncture and the top of the culture bottle (rubber cap) were cleaned thoroughly with 70% alcohol; the sample was obtained from each patient for aerobic blood culture. The culture bottles were incubated in a BACTEC 9050 blood culture machine (Becton Dickinson Diagnostics Sparks, Maryland, USA) at 35°C for a minimum of five days.
Biomarker measurement
Assay procedure for PCT: PCT levels were estimated by the enzyme-linked fluorescent assay (ELFA) technique using the commercially available VIDAS B.R.A.H.M.S PCT kit (BIOMERIEUX, Lyon, France). The PCT measurement range was 0.05–200 ng mL-1.
Assay procedure for quantitative QCRP: We used the latex agglutination technique with a Labmate semiautomated analyser (St. Albans, Hertfordshire, United Kingdom) for QCRP quantification.
Assay procedure for nCD64 and mHLA-DR: nCD64 and mHLA-DR were measured by a commercially available BD FACS CANTO system (Becton Dickinson, San Jose, California, USA) using a phycoerythrin (PE) fluorescence quantification kit (Quanti BRITEPE, Becton Dickinson). Blood for flow cytometry (FCM) analysis was collected in EDTA vials and stored at 2–8°C and processed within 4 h of collection. Whole blood (50 µL) was incubated for 30 min at room temperature with a combination of anti-CD14-FITC (clone MφP9), anti-CD64-PE (clone MD22), and CD45PerCP (clone 2D1) in Tube 1; and anti-HLA-DR PE (clone L243), anti-CD14 PerCP-Cy5.5 (clone MφP9), and CD45 APC H7 (clone 2D1) in Tube 2. After lysis of the red blood cells, the samples were washed and cells were resuspended in sheath fluid. The intensity of PE fluorescence on neutrophils and monocytes was determined to correspond to a minimum of 10,000 cells. Inter-assay standardisation for CD64 and HLA DR quantitation was performed using Quanti BRITE PE calibration beads with known numbers of PE molecules. The median fluorescence intensity (MFI) of the respective Quantibrite PE beads aids in calculating the antibody bound per cell (ABC) values for n-CD64 and mHLA-DR. Data analysis was performed using BD FACS Diva software. The optical and fluorescence characteristics of the target particles form the basis of FCM. The cells are made to pass one at a time through a laser beam. The forward scatter characteristics (FSC) of the particle are then measured using a forward light scatter detector and the side scatter characteristics (SSC) are measured using a side light scatter detector. The target cells can also be stained with a fluorochrome-conjugated antibody. The laser causes the fluorochrome to excite to a higher energy level, and when returning to the base energy level, the fluorochrome emits light energy typical for the fluorochrome, which is then measured by fluorescence emission detectors. The FSC depicts the size and SSC complexity of the cell, and the fluorescence intensities represent the expression of the molecules of interest by the cell. The blood cell populations can be separated based on their forward and side scatter characteristics as well as on the expression of certain molecules. Data from the flow cytometer are analysed using a computer program. The antigen expression can be reported as the percentage of antigen-positive cells; if there is a need to set a threshold for antigen positivity, it can be accomplished by isotype control-stained samples. A more accurate method is to report the results quantitatively as mean or median fluorescence intensity (MFI), which is the mean or median fluorescence intensity of the investigated antigen per cell.
IBM SPSS® software (version 21.0) was used for data analysis. P < 0.05 was considered with 95% CI (confidence interval) in the study. The difference in mean values and the χ2 test were used for categorical data; the paired t-test and independent Student’s t-test were used for non-parametric data; and logistic regression and receiver operating characte-ristics (ROC) curves were applied to compare the risk of death with the studied variables and specificity and sensitivity, respectively. Differences were considered significant at P < 0.05.
RESULTS
A total of 27 sepsis cases and 24 controls were included in this study. Subjects were divided into two groups (cases/sepsis and disease controls/non-sepsis) based on the clinical diagnosis and supported by the relevant culture.
Table 1 shows the age, distribution of sex (percentage of males), and mortality in cases and controls. The χ2 test was used to compare the two groups in terms of age and percentage of males; the groups were found to be comparable. Mortality was significantly higher among the cases than in the controls.
TABLE 1
Comparison of demographic parameters between cases and controls
Table 2 shows the number of infection sites among the cases and the diagnosis of patients included in the control group.
TABLE 2
Infection sites/diagnosis of cases and controls
Table 3 shows the comparison of SOFA score, TLC, and the levels of four biomarkers of sepsis on the first and third day between the cases and controls. We found that there was a significant difference between SOFA scores as well as serum PCT, QCRP, nCD64, and mHLA-DR levels on the first and third day of admission, between the case and control groups. TLC showed a statistically non-significant difference on days 1 and 3.
TABLE 3
Comparison of SOFA score, TLC and different biomarkers between the groups at first (D1) and third (D3) day of admission
Table 4 shows the comparison of changes in the SOFA score, TLC, and different biomarkers between the first and third day of admission in the case and control groups. There was no significant difference in the SOFA score, TLC, and levels of different biomarkers of sepsis (PCT, QCRP, nCD64, and mHLA-DR) on the first and third day of admission.
TABLE 4
Comparison of SOFA score, TLC and different biomarkers between first (D1) and third (D3) day of admission in different groups
Table 5 shows the predictive values of the SOFA score and the different sepsis biomarkers in detecting cases. We found that the SOFA score had the best predictive value followed by Sr PCT and nCD64, in detecting sepsis. The specificities of PCT, SOFA score, and nCD64 were 70.8, 79.2, and 70.8, respectively, whereas the sensitivities of PCT, SOFA score, and nCD64 were 77.8, 96.3, and 77.8, respectively. The positive predictive values (PPV) of the SOFA score, PCT, and nCD64 were 83.9, 75.0, and 75.0, respectively. Thus, serum PCT, SOFA score, and nCD64 can be used as diagnostic tools for the detection of sepsis. Based on the correlation between PCT and SOFA score (r = 0.001; r = Pearson correlation coefficient) and that between PCT and nCD64 (P = 0.032) (NS), it can be stated that both the parameters can be used in the diagnosis of sepsis; however, a statistically significant difference between PCT and nCD64 (P = 0.013) means that PCT is a better diagnostic tool than nCD64.
TABLE 5
Predictive values of different sepsis biomarkers in detecting cases, day 1
FIGURE 2
ROC curve showing sensitivity and specificity of SOFA and different biomarkers in predicting cases

Table 6 shows the comparison of study parameters on the day of admission between deceased and discharged patients. It is evident that low mHLA-DR, measured within 24 h of admission, is significantly associated with mortality. Total leukocyte count, SOFA score, and other biomarkers (serum PCT, QCRP, nCD64, and mHLADR) did not show any significant difference between the survivors and non-survivors.
TABLE 6
Comparison of study parameters at day of admission between expired and discharged patients
DISCUSSION
A review of the available literature revealed that this is the first study to evaluate the efficacy of SOFA score and four different biomarkers, viz. serum PCT, QCRP, nCD64, and mHLA-DR, assayed sequentially (day 1 and day 3 of sepsis), in the diagnosis and prognosis of patients with suspected sepsis and who were admitted to the ICU. We also determined the best cut-off value for each biomarker.
In our study, we found that PCT (cut-off of > 2.7 ng mL-1) correctly predicted sepsis with sensitivity, specificity, PPV, and negative predictive value (NPV) of 77.8%, 70.8%, 75.0 %, and 73.9%, respectively (Table 5). With a cut-off value of 2.25 ng mL-1, PCT was reported to have a sensitivity and specificity of 65.12% and 71.6%, respectively, in detecting sepsis [14]. A retrospective study found that the best cut-off value for PCT for diagnosis of sepsis was 1.1 ng mL-1 (sensitivity, 82%; specificity, 68%) [15]. An important fallacy with PCT is that it may be raised even in those patients who do not have sepsis [16]. In these non-septic cases, however, the plasma PCT levels are usually not very high (< 2 ng mL-1); the levels may increase under certain conditions such as surgery, trauma, burns, cardiogenic shock, multi-organ dysfunction syndrome (MODS), severe SIRS including severe viral infection, pancreatitis, heat stroke, severe liver or renal dysfunction, autoimmune disorders, end-stage tumour, and rhab-domyolysis [16]. Our control patients showed some of these conditions such as pancreatitis, postoperative phase, MODS, and liver and renal dysfunction (Table 2), which could explain the increased mean PCT level in this group (Tables 3 and 4).
In our study, there was no significant difference in serum PCT levels between survivors and non-survivors on day 1 (Table 6). Previously, it was reported that the mean PCT level did not seem to have a robust correlation with clinical outcome; the initial values were only marginally higher in the non-survivors than in those who survived [17]. Thus, although PCT may be useful in the diagnosis, it is a poor biomarker for predicting the prognosis of patients with sepsis.
We observed that the QCRP cut-off of > 65.44 ng dL-1 correctly predicted the occurrence of sepsis in the cases with sensitivity, specificity, PPV, and NPV of 70.4 %, 79.2%, 79.2%, and 70.4%, respectively (Table 5). In addition, we did not find any significant difference in QCRP levels between survivors and non-survivors (Table 6). A previous study also has reported that although the mean QCRP level was higher in patients who died than in those who survived, this difference was not significant [18].
Further, we observed that a SOFA score cut-off of > 2.5 correctly predicted the occurrence of sepsis in cases with sensitivity, specificity, PPV, and NPV of 96.3%, 79.2%, 83.9 %, and 95.0%, respectively (Table 5). The SOFA score was higher among both the non-survivor and survivor septic patients on day 1; however, this difference was not significant (Table 6). These findings related to the SOFA score were similar to those reported previously [19, 20]. In a study that evaluated the serum PCT level and SOFA score at discharge from the ICU to predict post-ICU mortality, it was observed that those who died had a higher SOFA score and serum PCT at the time of discharge from the ICU as compared to those who survived [19]. Similar findings have also been reported among ICU patients [20]. However, when patients developed unresolvable coma or chronic renal failure, the SOFA score remained persistently elevated, thus making it an unacceptable indicator for deciding discharge from the ICU. Moreover, the SOFA score does not include parameters regarding infection or malnutrition; in our study as well, many of the patients with renal dysfunction, liver diseases, and unresolvable neurological diseases were still discharged from our ICU with higher SOFA scores [19].
The nCD64 was significantly higher among the cases than in controls, on both day 1 and day 3 (Table 2); however, no significant change in mean nCD64 level was observed between day 1 and day 3 (Table 4). Nonetheless, the mean change in nCD64 level from day 1 to day 3 was observed to be higher among septic patients than that in controls (Table 4).The nCD64 cut-off of > 985.5/cell correctly predicted sepsis in the cases with sensitivity, specificity, PPV, and NPV of 77.8 %, 70.8%, 75.0 %, and 73.9%, respectively (Table 4). The nCD64 levels were higher in non-survivors than those in survivors on both days 1 and 3 (Table 3). There was no significant difference in the levels of nCD64 between non-survivors and survivors among patients with sepsis (Table 6). A recent study has also reported that there was no difference in mean nCD64 levels between survivors and non-survivors on days 0 and 4; however, a significant difference was observed on day 8 [21]. In our study, we found that nCD64 levels were distinguishable between sepsis(cases) and non-sepsis patients (disease controls). The nCD64 level was not a good predictor of mortality between non-survivors and survivors among patients with sepsis. A previous study has reported that the diagnostic performance, as gauged by the clinical score, varied with nCD64 (sensitivity 87.9%, specificity 71.2%, efficiency 76.8%) and outperformed QCRP (sensitivity 88.2%, specificity 59.4%, efficiency 69.4%) [22]. In the aforementioned study, the PCT levels were not estimated. In our study, we did estimate PCT levels and found PCT to be a better biomarker than nCD64 for the detection of sepsis. In another previous study, it was reported that nCD64 levels were significantly higher in the patients with bacterial infection than those in patients with viral infection and patients in the control group [23].
The authors of a previous study reported the utility of the CD64 index in the detection and management of sepsis and severe bacterial infections [24]. Using a cut-off of > 1.19, they found that this index is highly sensitive and specific for the final diagnosis of infection. Furthermore, a CD64 index of ≤ 1.19 was a predictor of a negative blood culture result and could identify false-positive results. A recently published meta-analysis compared the accuracy of nCD64, procalcitonin, and CRP for sepsis identification [20]. It was reported that the pooled sensitivity and specificity of nCD64 for diagnosing infection in adult patients with septic syndrome were 0.87 and 0.89, respectively. They found that in adult patients with septic syndrome, nCD64 level is an excellent biomarker with moderate accuracy, which is better than that of either QCRP or PCT [20].
To mount an appropriate immune response to an infection, it is essential that class II major histocompatibility complexes (MHCs) are expressed on monocytes for effective antigen presentation and processing [25]. In the present study, we also found that mHLA-DR expression decreased in septic patients and increased in non-sepsis/disease control patients. When we compared the mHLA-DR between the groups on day 1 and day 3, we found that mHLA-DR level was significantly lower among septic patients than that in controls on both days (Table 3). No significant change in the mean mHLA-DR level was observed between days 1 and 3 (Table 3); nonetheless, the change was larger among the septic patients than that in controls (Table 4). The mHLA-DR with a cut-off of <5000 correctly predicted the occurrence of sepsis in cases with sensitivity, specificity, PPV, and NPV of 29.9%, 25.0%, 76.9%, and 72.0%, respectively (Table 5). The mHLA-DR expression on day 1 was significantly lower among the non-survivors than that in the survivors (Table 6). Thus, lower mHLA-DR level at admission could be a marker of poor outcomes in patients with sepsis. Among patients who underwent elective major resection surgery, the percentage of HLA-DR- positive monocytes decreased significantly on the first postoperative day [25]. The mHLA-DR level was significantly higher in patients without sepsis than in those with sepsis on days 1, 3, and 5. The authors believed that these findings have important clinical implications and mHLA-DR could have predictive value for biological response modification in patients at risk of developing sepsis after surgery [25]. In another study, it was reported that changes in mHLA-DR, ΔmHLA-DR3 (change in mHLA-DR level on day 3 compared with the level on day 0), and ΔmHLA-DR7 (change in mHLA-DR level on day 7 compared with that on day 0) were reliable indicators of mortality in patients with severe sepsis [11]. We also found that lower mHLA-DR level within 24 h of admission was significantly associated with mortality (Table 6). In a study of ICU patients, it was reported that mHLA-DR limits of 2,000 and 5,000 molecules/cell were able to discriminate patients with longer ICU stay, ventilation time, and duration of antibiotic therapy as well as higher counts of microbiological findings from those who had a more benign course [25]. Moreover, the mHLA-DR value of ≤ 2,000 molecules/cell was linked to a higher requirement for overall antibiotic therapy [26].
A literature search did not reveal many studies that compared the utility of different biomarkers in the diagnosis and prognosis of patients with sepsis. A recent systematic review and meta-analysis appraised the role of nCD64 in the diagnosis of sepsis in adults and compared it with that of QCRP and PCT. They reported that the area under the receiver operating characteristic (ROC) curve was greater for nCD64 than that for QCRP (0.89 vs. 0.84) or PCT (0.89 vs. 0.84) and hence concluded that in adult patients with septic syndrome, nCD64 is a reliable biomarker with moderate accuracy, which is better than that of both QCRP and PCT [20]. In our study, in addition to the SOFA score, the sensitivity and specificity of serum PCT and nCD64 showed the best values among the four analysed biomarkers, for the diagnosis of sepsis (Table 5). In a recent pilot study, flow cytometric characteristics of blood samples taken at admission were evaluated for their value in predicting the prognosis of patients with sepsis, and it was found that mHLA-DR and CD64 can be used as markers for predicting mortality in critically ill septic patients with acceptable sensitivity and specificity (CD64 had a sensitivity of 67% and specificity of 82%, whereas mHLA-DR had a sensitivity of 76% and specificity of 67%) [27].
We understand that the present study has an important limitation. As the sample size was small, a comprehensive analysis of relationships between different biomarker levels and disease characteristics (for example, infecting organism, source of sepsis, culture results correlation) or severity of sepsis (for example, severity score, multiorgan failure) as well as therapy instituted was precluded.
The present study was a preliminary single-centre longitudinal cohort study. Thus, because of the above stated limitations, we suggest that the results of the present study need to be corroborated by a larger multicentric, observational, prospective study with consideration of the above-mentioned limitations.
CONCLUSIONS
Serum PCT and nCD64 are considerably effective biomarkers for the detection of sepsis; their detection capacities are comparable. mHLA-DR could play a role in prognosis as lower levels of this biomarker on day 1 were associated with higher mortality. Subsequent testing for any of the analysed biomarkers on day 3 did not add to the diagnostic or prognostic value.