Introduction
Allergic conjunctivitis has become one of the most common non-traumatic extraocular inflammatory diseases [1–5]. About 90% of allergic conjunctivitis are vernal conjunctivitis and spring catarrh [6]. It has the features of seasonal (usually in summer rather than spring) basis and occurs after seasonal rhinoconjunctivitis in adults and children with the family history of atopy [7–9]. Grass, tree and weed pollens and outdoor moulds are the common factors to cause allergic conjunctivitis [10]. The clinical manifestations include recurrent bilateral conjunctivitis which result in itching, redness, lacrimation, burning, stinging, photophobia and watery/mucoid discharge, which are accompanied by clinical signs of lid oedema, conjunctival chemosis, hyperaemia and papillary reactions [11, 12].
Current treatment methods for allergic conjunctivitis aim to prevent and alleviate symptoms by using allergen elimination, cold compression, artificial tears, modulation of the immune system and pharmacological inhibition of chemical mediators involved in the immune response such as topical anti-histaminics, nonsteroidal anti-inflammatory drugs (NSAIDs), mast cell stabilizers and steroids [13–17]. However, these methods were limited by insufficient efficacy or adverse effects such as conjunctival hyperaemia, corneal stinging and burning [6].
New generation multiple action topical antiallergic agents such as olopatadine and ketotifen are recommended as the first-line agents in the treatment of allergic conjunctivitis, but their efficacy and safety are not well compared [18, 19].
Aim
We therefore conducted this meta-analysis of RCTs to evaluate the effectiveness and safety of olopatadine versus ketotifen on treatment efficacy and safety for allergic conjunctivitis.
Material and methods
Study selection and data collection
This meta-analysis of previous studies did not need ethical approval and patient consent and was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement and Cochrane Handbook for Systematic Reviews of Interventions [20, 21].
We have searched PubMed, Embase, Web of Science, EBSCO and the Cochrane library for articles published up to November 2022, using the search terms “conjunctivitis” AND “olopatadine” AND “ketotifen”. The inclusion criteria were as follows: (1) study design was RCT; (2) patients were diagnosed with allergic conjunctivitis; and (3) intervention treatments were olopatadine eye drops versus ketotifen eye drops.
Quality assessment
The Jadad Scale was used to evaluate the methodological quality of individual RCT [22]. This scale consisted of three evaluation elements: randomization (0–2 points), blinding (0–2 points), and dropouts and withdrawals (0–1 points). The score of Jadad Scale varied from 0 to 5 points. Jadad score ≤ 2 suggested low quality, while Jadad score ≥ 3 indicated high quality [23].
Outcome measures
The following information was extracted: first author, publication year, sample size, age, weight, male and methods of two groups. The primary outcomes were hyperaemia and itching. Secondary outcomes included tearing and papillae.
Statistical analysis
A team consisting of three authors did the statistical analyses. Mean difference (MD) with 95% confidence interval (CI) was used to assess continuous outcomes. I 2 statistic was used to assess the heterogeneity, and significant heterogeneity was observed when I2 > 50% [24]. The random-effect model was used when encountering significant heterogeneity, and otherwise a fixed-effect model was applied. We conducted the sensitivity analysis through detecting the influence of a single study on the overall estimate via omitting one study in turn or using the subgroup analysis. P ≤ 0.05 indicated statistical significance and Review Manager Version 5.3 was used in all statistical analyses.
Results
Literature search, study characteristics and quality assessment
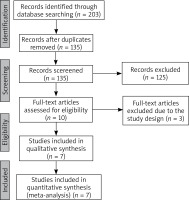
The flowchart for the selection process and detailed identification was presented in Figure 1. 203 publications were identified through the initial search of databases. Ultimately, seven RCTs were included in the meta-analysis [6, 25–30].
The baseline characteristics of the seven eligible RCTs in the meta-analysis were summarized in Table 1. The seven studies were published between 2005 and 2022, and total sample size was 449. There were similar baseline characteristics between the olopatadine group and the ketotifen group. Olopatadine eye drops were administered at a concentration of 0.1%, while ketotifen eye drops were administered at a concentration of 0.025% or 0.05%.
Table 1
Characteristics of included studies
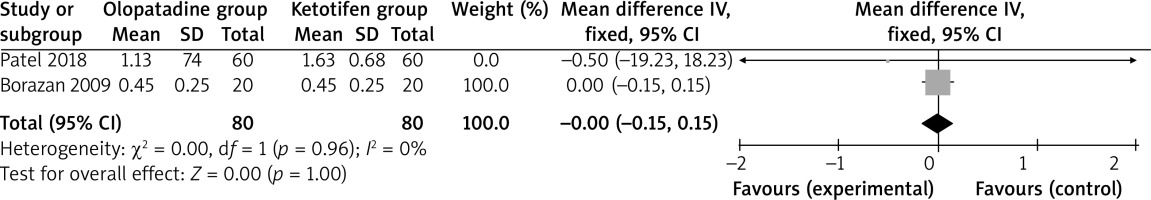
Among the seven RCTs, two studies reported hyperaemia [6, 26], three studies reported itching [6, 26, 29], two studies reported tearing [26, 29] and two studies reported papillae [6, 26]. Jadad scores of the seven included studies varied from 3 to 5, and all studies were considered to be high-quality ones according to quality assessment.
Primary outcomes: hyperaemia and itching
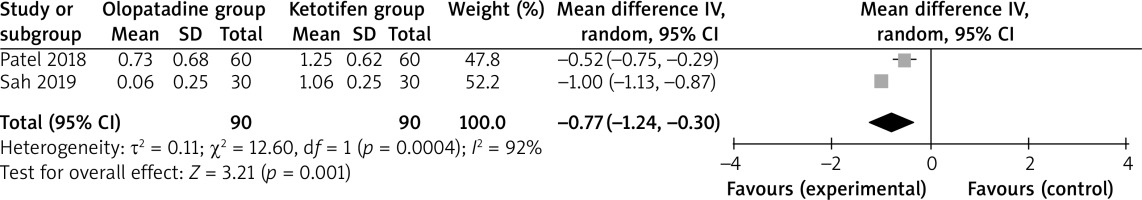
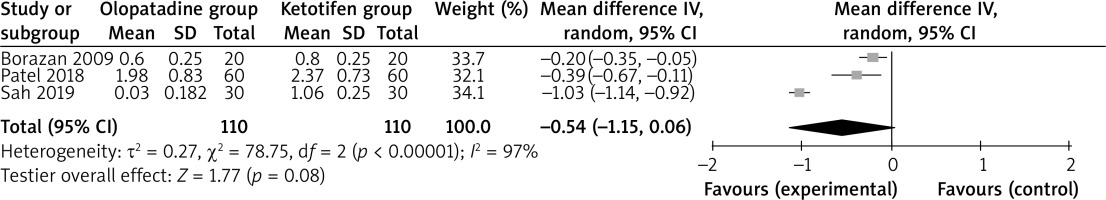
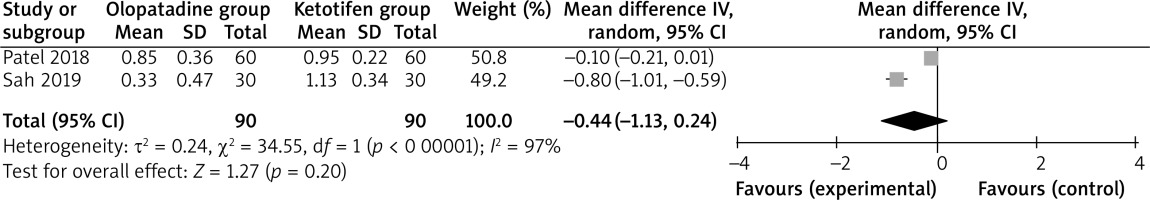
Compared to ketotifen intervention for allergic conjunctivitis, olopatadine intervention results in significantly lower hyperaemia (MD = –0.77; 95% CI = –1.24 to –0.30; p = 0.001) with significant heterogeneity among the studies (I2 = 92%, heterogeneity p = 0.0004, Figure 2), but showed comparable itching (MD = –0.54; 95% CI = –1.15 to 0.06; p = 0.08) with significant heterogeneity among the studies (I2 = 97%, heterogeneity p < 0.00001, Figure 3).
Sensitivity analysis
Significant heterogeneity was observed for the primary outcomes. Only two studies were included in Figure 2, and thus we did not perform the sensitivity analysis by omitting one study in turn for the meta-analysis. As shown in Figure 3, the study conducted by Sah showed results that were almost out of range of the others and probably contributed to the heterogeneity [6]. After excluding this study, the results suggested that olopatadine intervention was associated with substantially reduced itching compared to ketotifen (MD = –0.26; 95% CI = –0.43 to –0.09; p = 0.003), and only heterogeneity remained low (I2 = 26%, p = 0.24).
Discussion
In order to compare the efficacy and safety of olopatadine with ketotifen for patients with allergic conjunctivitis, our meta-analysis included seven RCTs and 449 patients. The results suggested that compared to ketotifen intervention, olopatadine treatment was able to significantly reduce the severity of hyperaemia, but showed no obvious impact on itching, tearing or papillae.
Regarding the sensitivity analysis, there was significant heterogeneity for the primary outcomes. After excluding the study conducted by Sah [6], the results suggested that olopatadine intervention could substantially reduce itching compared to ketotifen (p = 0.003), and only heterogeneity remained low (I2 = 26%, p = 0.24). These results suggested that olopatadine intervention was superior to ketotifen for relieving the symptoms of allergic conjunctivitis. The significant heterogeneity may be caused by the different concentrations of ketotifen and treatment durations. In addition, olopatadine was reported to provide quicker relief of symptoms, improved quality of life and fewer side effects than ketotifen for patients with allergic conjunctivitis [26].
The pathological factors of allergic conjunctivitis were complex, and mainly included genetics, air pollution, pets and immune responses [5, 15, 31]. Seasonal and perennial conjunctivitis occurred after the exposure to specific allergens, during which mast cells were activated by IgE antibodies [32]. The response of allergic conjunctivitis to nonspecific allergens were regulated by Th2 cells, mast cells and eosinophils [33]. As one selective histamine H1 receptor antagonist and mast-cell stabilizer, olopatadine hydrochloride had an important anti-inflammatory effect including suppression of interleukins (IL)-6 and 8 by inhibiting histamine related-signalling pathways [34, 35].
We should also consider several limitations. Firstly, our analysis was based on seven RCTs and more studies with larger patient samples should be conducted to confirm our findings. Secondly, there was significant heterogeneity, which may be caused by different concentration and treatment durations of two drugs. Thirdly, allergic conjunctivitis with different levels of severity were included in this meta-analysis, which may affect the efficacy assessment.