Introduction
Alopecia areata (AA) is a form of non-scarring alopecia, which occurs in approximately 1–2% of the general population, equally affecting both sexes [1, 2]. It is estimated that the disorder is one of the most common causes of hair loss. It usually affects children and young people between the second and third decade of life. Alopecia areata typically involves well-demarcated areas on the scalp, with one to several bald patches. In more severe cases a generalized pattern of total body hair loss may occur [2, 3]. It is believed that 30–50% of patients experience hair regrowth within a year following the onset of the first episode, whereas 5% of cases progress to alopecia totalis and 1% to alopecia universalis [3–5]. Factors that negatively affect the prognosis include: prolonged and extensive course of the disease, an ophiasis pattern of hair loss, an early age of onset, a family history, nail changes, comorbid autoimmune disorders (including Hashimoto’s disease) or atopy [1–4]. The etiopathogenesis of AA is still unclear. Undoubtedly AA is an autoimmune condition, but genetic and environmental factors also play a substantial role [1–5]. The infiltration of Th1 and Th2 cells producing a range of cytokines may be observed in the hair follicle area. It seems that the major role is played by Th1 lymphocytes and the cytokines they produce. Numerous studies revealed the increased concentration of Th1-derived interleukins, including interleukin (IL) 12 (IL-12), IL-6 or tumor necrosis factor α (TNF-α) in the blood serum of patients, which confirms that Th1 lymphocytes play a role in the etiopathogenesis of AA. It seems that IL-17, IL-22 and IL-23 produced by Th17 lymphocytes also contribute substantially to the development of the disease. Furthermore, a number of available studies suggest the role of Th2 lymphocytes and the reduced activity of cytokines they produce, including IL-10 [4, 5]. Thus, the two major factors contributing to AA etiopathogenesis are the activation of the immune system as well as the disturbed interaction between Th1 and Th2 lymphocytes.
Aim
Evaluation of the immune response associated with Th1, Th2 and Th17 cells in people with AA. To this end, the concentrations of IL-10, IL-12, IL-17 and IL-35 in blood serum of patients were measured and compared to healthy controls and juxtaposed with such factors as the severity of AA, comorbidity of thyroid diseases and atopy, as well as sex and age of patients.
Material and methods
The study group consisted of 118 patients with AA, including 76 women and 42 men. The mean age of the group of women was 37.8 ±12.6 years and that of the group of men was 30.1 ±12.3 years. The mean disease duration was 109.1 ±132.5 months, shorter in men (73.0 ±104.2 months) than in women (129.0 ±142.5 months). The average age at disease onset was 25.2 ±15.1 years. Women had on average 2.5 ±2.3 disease episodes (defined as hair loss and complete regrowth) whereas the mean number of episodes in men was 1.4 ±0.8 (p = 0.005). The SALT score was used to determine the extent of hair loss. The percentage of hair loss was assessed in four areas: occipital – A, parietal – B, right side – C and left side – D. Then, the percentage of scalp hair loss was calculated according to the following formula: % of hair loss = (A × 0.24) + (B × 0.4) + (C × 0.18) + (D × 0.18) [6, 7]. The mean percentage of hair loss in the study group was 49.6 ±36.3%, including 48.4 ±36.8% for women and 51.6 ±35.7% for men (p = 0.651). Subsequently, patients were divided into 5 groups based on the severity of hair loss, according to the following formula: SALT 1 – hair loss up to 25%, SALT 2 – 25–49% loss, SALT 3 – 50–74% loss, SALT 4 – 75–99% loss, SALT 5 – 100% hair loss. SALT 1 group consisted of 44 (37.9%) patients, SALT 2 – of 22 (19.0%) patients, SALT 3 – of 14 (12.1%) patients, SALT 4 – of 7 (6%) patients, and SALT 5 – of 29 (25%) patients. Among the study group, AA was associated with comorbid atopic disorders in 23.3% of patients. Autoimmune thyroid disease was diagnosed in 24.1% of patients, more frequently in women (36%) than in men (2.4%) (p < 0.001). Eyebrow hair loss occurred in 37.1% of subjects, eyelash hair loss in 34.5%, hair loss in axillary and pubic areas occurred in 27.6% of patients. Nail changes (pitting, vertical nail ridges) were found in 17.6% of the subjects. The control group consisted of 54 healthy individuals, aged 24–80 (mean: 37 ±11.5 years), including 25 men and 29 women. Blood was collected from patients and controls to assess serum levels of IL-10, IL-12, IL-17 and IL-35. Assays were performed by enzyme-linked immunosorbent ELISA by means of Quantikine® ELISA kits (R&D Systems®, USA) and EIAab® (Wuhan, China). The tests were carried out in accordance with the reagent manufacturers’ protocols and the principles of good laboratory practice. The concentration of cytokines in serum samples was measured by determining their optical density using an EPOCH microplate spectrophotometer (BioTEK® Instruments, Inc., USA). The measurement was carried out at a wavelength of 450 nm and at a reference wavelength of 540 nm. The concentration values for individual samples were determined on the basis of standard curves created automatically by Gen5® software.
Statistical analysis
The nominal and ordinal variables were presented as amount (n) and proportion (%). A Pearson’s χ2 (chi-square) test was used to assess the independence of two qualitative variables. Mean values (M), standard deviations (SD), median (Me), first and third quartiles (Q1, Q3) as well as extreme values (min., max.) were calculated for quantitative variables. Compliance between quantitative variables distribution and normal distribution was measured by the Shapiro-Wilk test. Depending on the result of the normality test, the mean values of quantitative variables in two independent groups were compared either by the Student’s t-test or by the Mann-Whitney U test. Analysis of variance (ANOVA) or Kruskal-Wallis test was used to compare mean values (M or Me) in three or more groups. Spearman’s rank correlation coefficient was used to assess the correlation between groups. Statistical significance was determined at the level of p < 0.05. Calculations were performed using Statistica 12.5 software (StatSoft, Inc., USA).
Results
In the group of AA patients, IL-12 level was 10.50 ±4.57 which turned out to be significantly higher than in the control group, where it amounted to 8.84 ±3.96 (p = 0.023). Accordingly, the level of IL-17 in the serum of patients was 0.31 ±0.35 and was significantly increased compared to the control group (0.16 ±0.07) (p = 0.003). The level of IL-10 in the study group was slightly higher (0.46 vs. 0.41; p > 0.05) and the level of IL-35 slightly lower than in the control group (14.65 vs. 18.43; p > 0.05), however those differences were not statistically significant (Table 1).
Table 1
Concentrations of selected interleukins in the group of patients and in the control group
Patients with severe disease classified to groups SALT 2 – SALT 4 (hair loss 25–99%) had elevated IL-12 levels (11.9 ±3.8) and decreased IL-35 levels (9.2 ±12.2) compared to patients of SALT 1 and SALT 5 groups with the lowest and highest disease severity (p < 0.05) (Table 2).
Table 2
Concentrations of selected interleukins vs. severity of the disease
Levels of IL-10, IL-17 and IL-35 in both men and women with AA were comparable (p > 0.05), whereas IL-12 level in women was significantly higher than in men (11.22 vs. 9.20, p = 0.025) (Figure 1).
Figure 1
Concentration of IL-12 in blood serum of women and men. IL-12 level is significantly higher in women than in men (p = 0.021)
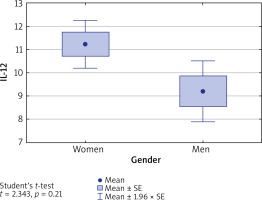
The concentration of IL-12 in the group of women with AA was not affected by the presence of autoimmune thyroid disease (mean: 12.1 ±4.7) or its absence (10.9 ±4.4) (p = 0.392).
Likewise, concentrations of IL-12 in the group of all patients with AA were also unaffected by the presence of thyroid disease (11.7 ±5.0) or its absence (10.2 ±4.4) (p = 0.198).
However, the level of IL-12 in the group of patients with AA but without thyroid disease (10.2 ±4.4) was found to be higher than in the control group (8.8 ±4.0). The result was on the verge of statistical significance (p = 0.094).
It was also found that in patients diagnosed with some atopic conditions, the IL-12 level was lower than in patients without any allergy (p = 0.001) (Figure 2).
Figure 2
Concentration of IL-12 vs. coexistence of allergies. IL-12 level is significantly higher in patients without allergies (p = 0.001)
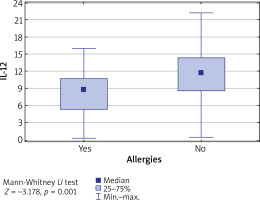
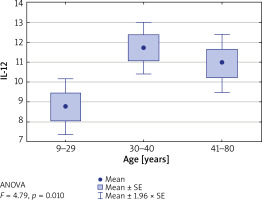
Furthermore, the concentrations of tested interleukins were evaluated in relation to patients’ age, divided into the following ranges: up to 29 years, between 30 and 40 years and between 41 and 80 years. It was found that in patients aged 30–40 years, the IL-12 level was significantly higher than in other age groups (p = 0.01) (Table 3). Similarly, the level of IL-12 was the highest in the group of patients who suffered from the first episode of the disease between the age of 15 and 33 (p = 0.009) (Figure 3).
Table 3
Concentrations of selected interleukins vs. patients’ age
Figure 3
Concentration of IL-12 vs. patients’ age. IL-12 level is significantly higher in patients over 30 years old (p = 0.01)
Taking into consideration the number of hair loss episodes over the course of the disease, there were no statistically significant differences in the concentration of IL-10, IL-12, IL-17, IL-35 (p > 0.05) within the examined groups of patients. Accordingly, there were no differences in the concentration of interleukins in relation to the duration of the disease (p > 0.05).
Discussion
The etiopathogenesis of AA is still not fully understood. Lymphocytic infiltration around hair follicles consists of both CD4+ and CD8+ cells. Although it seems that the key role is played by Th1 lymphocytes, the increased concentrations of interleukins produced both by Th1 and Th17 in blood serum and infiltration around hair follicles indicate the complexity of the disease process [1–5]. Studies have shown that the elevated concentration of IL-12 is found in many autoimmune diseases [8–10]. In some diseases, including multiple sclerosis, rheumatoid arthritis and thyroid disease, IL-12-dependent lymphocyte differentiation into Th1 involved in the development of the autoimmune process was observed [8, 11]. IL-12 is also implicated in the pathogenesis of psoriasis what has been used in the targeted therapy with anti-IL-12/23 antibody (ustekinumab) [12]. Interleukin 12 is a heterodimer composed of a 35 kDa α chain and a 40 kDa β chain and produced mainly by dendritic cells and macrophages. The IL-12 secretory pathways are activated by such stimuli as the presence of pathogens. Dendritic cells are activated in the skin and migrate to regional lymph nodes where stimulation and activation of T-cells occurs. Interleukin 12 is responsible for the Th1 line differentiation. Furthermore, through its receptor IL-12R (composed of β1 and β2 subunits), IL-12 also affects NK cells, leading to interferon γ (IFN-γ) synthesis [8–10]. Therefore, IL-12 not only plays a role in the immune response to infection, but also modulates autoaggressive reactions mediated by Th1 lymphocytes [7, 13]. This study revealed a higher level of IL-12 in patients compared to the control group (p = 0.023), which proves that IL-12 is involved in the etiopathogenesis of AA, considered to be an autoimmune disease. Increased concentrations of this cytokine in the serum of AA patients have also been demonstrated by Rossi et al. [4] and Barahmani et al. [14], confirming that Th1 plays a role in the development of the autoimmune process [13]. The complex role of IL-12 in the development of AA is also observed through reactions to IL-12 inhibitor administration (ustekinumab, IL-12/23 antagonist). In the world literature, there are reports of the effective use of ustekinumab in AA in a patient with concomitant AA and psoriasis. Hair regrowth in this case may prove the role of IL-12 in immunopathogenesis of this kind of alopecia [15]. On the other hand, lack of the effectiveness or even stimulation of the hair fall in the course of ustekinumab therapy administered for concomitant psoriasis was also described in three cases, which suggests a more complex role of IL-12 in AA [16–18]. Our study demonstrated higher IL-12 levels in women (p = 0.026) than in men, which is probably due to the fact that autoimmune diseases are more prevalent in women. Twenty seven female patients with AA had a comorbid autoimmune thyroid disease. However, differences in IL-12 levels with regard to the coexistence of thyroid disease in the group of women with AA were not statistically significant (p = 0.392). Elevated levels of IL-12 were also found in AA patients of both sexes without comorbid thyroid disease (88 individuals), as compared to the control group (54 individuals), and the difference remained on the verge of statistical significance. It seems that the increased IL-12 level was associated with the immunological process leading to the development of AA as it was found by other researchers in the pathogenesis of autoimmune diseases such as thyroid diseases. Furthermore, in the study group elevated IL-12 levels were observed in people aged 30–40 (p = 0.010), compared to people below 29 and above 41 years, which may indicate active autoimmune processes in young adults. Regarding the extent of hair loss, it was demonstrated that IL-12 concentration was the highest in people with a loss of 25–99% (p = 0.032). In the case of patients with either alopecia totalis or mild disease, the level of IL-12 was lower, including the lowest in patients with alopecia totalis. Perhaps the immune processes stimulated by the hair building components are less intense after total hair loss. The reduction of IL-12 found in AA patients with comorbid atopy (p = 0.001) corroborates the findings of other researchers, who demonstrated decreased levels of this cytokine in the course of atopic diseases, including asthma, indicating that allergic processes are dominated by the Th2 lymphocytes-related immunological processes [10, 11, 13, 15, 19].
The processes associated with Th17-cell activity also play a role in the development of autoimmune diseases. Interleukin 17, which is mainly produced by Th17 lymphocytes and, to a lesser extent, by Tregs, NK cells and neutrophils, stimulates cells to produce other inflammatory agents: interleukin 1β (IL-1β), IL-6, IL-8, TNF-α, CXCL-1 and CXCL-6. It also affects the activation and migration of mainly neutrophils and the development of a rapid inflammatory response [20]. It plays a role in the etiopathogenesis of inflammatory and autoimmune diseases such as psoriasis, rheumatoid arthritis or Crohn’s disease [21, 22]. An increase in the IL-17 concentration as well as a higher concentration of Th17 cells also in the blood serum of AA patients have been demonstrated in the literature and potentially points to new therapeutic options using IL-17 inhibitors [20–24]. Our study also showed a statistically significantly higher concentration of IL-17 in the serum of patients compared to the control group (p = 0.003), which is corroborated by other scientific reports and substantiates the role of this cytokine in the etiopathogenesis of AA. Like other researchers, we did not find the concentrations to be correlated with the duration of the disease, the number of episodes or the severity of hair loss assessed by the SALT scale [23, 25–29]. Furthermore, we decided to investigate the level of IL-10 and IL-35 cytokines, which, according to the latest research, may inhibit the Th1 response and, if deficient, contribute to the development of autoimmune diseases. Interleukin 35 belongs to the IL-12 family and is secreted by Tregs, Bregs, dendritic cells, as well as endothelial cells and monocytes [30–32]. It is known to suppress the Th1- and Th17-dependent immune response [11, 31]. Elevated concentrations of this cytokine were found in inflammatory and cancer diseases, including acute myeloid leukaemia and lung cancer [31, 32], whereas reduced levels of IL-35 were observed in autoimmune diseases, such as inflammatory bowel diseases, type I diabetes and psoriasis [33–36]. Our study also revealed a lower level of IL-35 in the blood serum of patients with AA compared to healthy controls, however the difference was not statistically significant (p = 0.229). It also showed that in the group of patients with 25–99% hair loss, the level of IL-35 was lower than in the group of patients with alopecia totalis and with hair loss below 25%, i.e. in the same group of patients with an increased level of IL-12. Thus, suppressing the production of IL-35 with its ability to inhibit the Th1 response is likely to play a significant and maybe even a key role in the pathogenesis of the active phase of AA.
Interleukin 10 is produced by stimulated T cells (mainly Th2 and Treg), B cells, eosinophils, macrophages, monocytes, mast cells and keratinocytes. IL-10 is considered an anti-inflammatory agent, inhibiting the production of various cytokines produced mainly by Th1, i.e., IL-2, IL-3, IFN-γ. The immunosuppressive and anti-inflammatory activity of IL-10 is still under investigation and the results are ambiguous. Some research showed that IL-10-deficiency induces the development of some autoimmune diseases, such as rheumatoid arthritis and systemic lupus, as well as psoriasis. Our study found comparable concentrations of IL-10 in patients and in healthy controls, which was consistent with the observations of Barahmani et al. [14]. The impact of IL-10 on the development of AA requires further research [14, 30].
Conclusions
Although the obtained results indicate a complex etiopathogenetic process of AA, IL-12 and IL-17 are undoubtedly significant contributors in this process as evidenced by their elevated serum levels in patients. It seems that the key role is played by Th1 and Th17 lymphocytes and their increased activity. On the other hand, low levels of cytokines inhibiting inflammatory processes, such as IL-35, may underlie the development of the AA autoimmune process. Disorders of immunological processes in AA require further research in order to understand the underlying pathomechanisms of the disease and to provide potential therapeutic strategies.








