Critical care patients have a high demand of central nervous system (CNS) drugs for treating both primary disease as well as secondary complication [1]. Mechanical ventilation, intubation, suction and other interventions in intensive care unit often cause pain and tension that need analgesics as well as sedatives [2]. Additionally, alternative CNS agents are also utilized amongst critical care patients to treat anxiety, seizure, psychotic problems and surgery [1, 2]. The administration of sedation as well as analgesia has been proven to reduce the duration of mechanical ventilation and the length of stay in the intensive care unit [3]. The one novel approved analgesic injection is acetaminophen, which effectively reduces non-infectious fever, but has lower potency as an analgesic. Therefore, acetaminophen is mostly used in combination with other central analgesics.
The use of a combination of medicines is unavoidable in a critical condition. Since the patients often have limited venous access to deliver all of the intravenous medications, the use of a single lumen catheter for some concurrent medications is common [4]. This leads to delivery of several intravenous drugs including acetaminophen, which possibly has contact with other drugs through a three-way stopcock or infusion connector, i.e. Y-site. In terms of Y-site administration, the dwelling time is often less than 10 minutes; other authors reported that the Y-site minimum contact time is 2 minutes [5]. Therefore, the problem of incompatibility between acetaminophen and one or more drugs in a different bag or line often is a physical matter, rather than chemical. This means the evaluation of physical compatibility is significant in preventing the incompatibility problem at the Y-site.
There is limited research which refers to the compatibility of intravenous acetaminophen with alternate intravenous medications [6]. Lack of data on physical compatibility often urges nurses to attain extra-venous access. This will bring consequences for workload, infection and cost. Hence, research on compatibility of acetaminophen with CNS medications in critical care, where Y-site administration is frequently used, is needed. Furthermore, compati-bility of acetaminophen with alternative CNS agents used in critical care has not been studied yet. These are dexketoprofen, diazepam, fentanyl, haloperidol, ketorolac, ketamine, metamizole, midazolam, pethidine, phenytoin, phenobarbital, propofol, rocuro-nium, atropine sulfate and tramadol. This study aims to report the physical compatibility of acetaminophen with these fifteen selected CNS agents which often encounter acetaminophen.
METHODS
All medications were obtained from hospital stock as shown in Table 1. The manufacturer (Finusol-prima, Indonesia) supplied acetaminophen injection 10 mg mL-1 in a glass bottle of 100 mL. Diluent of glucose 5% was supplied by Otsuka. The medications, primarily antibiotics, were reconstituted with proper diluent following the manufacturer’s recommendation. Some medications were diluted according to the literature to yield lower concentration and possibly to deliver through continuous infusion. All preparations were done under aseptic conditions and sterile syringes and clean glass tubes in triplicate were used to make sure of the reproducibility. To confirm the incompatibility justification of mixed medication, acetaminophen was directly withdrawn and kept as a control solution in a glass tube.
TABLE 1
Drug solution tested compatibility with acetaminophen 10 mg mL-1
Based on the Allen method, compatibility evaluation was performed using a simulated Y-site with maximum concentration and volume ratio 1 : 1 of acetaminophen (10 mg mL-1) with an alternative intravenous medication, then the mixture was kept in a glass tube [7]. Each aliquot mixture was collected on each mixed solution and assessed at 0, 1, 4, and 24 hours for its visual and microscopy. All samples were maintained at ambient temperature in a laboratory (24–28°C). Physical incompatibility was identified as discoloration, turbidity, haze, visual particulate matter or precipitation.
Visual inspection was used to identify the color changes, turbidity, haze or visual particulate matter. Clarity was evaluated with the unaided eye in normal light by comparing the solutions against a light and dark background. To evaluate the visual changes, inspection was conducted by two independent trained people. A green laser pointer was used as Tyndall beam to magnify the ability of the naked eye to detect particles.
The particle confirmation and quantification were performed using a microscope (Olympus CX21). In order to increase the resolution and the brightness, immersion oil was applied. A 1 mL aliquot part solution was infused through a 0.45-µm nitrocellulose filter disc. Each disc was examined under 10–100× magnifications for precipitation identification. According to the USP 788, the amount of particles in aliquot sterile solution is considered physically compatible if it is less than 2 particles mL-1 measuring 25 µm, and less than 12 particles mL-1 with size 10 µm in diameter. Physical incompatibility of a particular lipid formulation or suspension as propofol is defined using microscopy as globule size more than 5 µm [8].
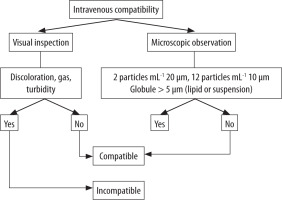
Justification of physical incompatibility refers to the diagram in Figure 1 [9]. The mixture was considered incompatible when there was a visual change and/or relevant particles (over the USP limit).
RESULTS
Acetaminophen showed compatibility with halo-peridol, ketamine, midazolam, pethidine, pheno-barbital, rocuronium, and tramadol during 24 hours, since there is no visual change or particle formation. Table 2 shows that incompatibilities between aceta-minophen and alternative medication occurred in every period of time.
TABLE 2
Physical incompatibility of mixture of acetaminophen and other central nervous system medication in ratio 1 : 1
TABLE 3
The pH changes of mixture of acetaminophen and other central nervous system medication in ratio 1 : 1
According to the Allen and Trissel method, Y-site simulation incompatibility was justified at 0, 1, and 4 hours. It was found that acetaminophen is physically incompatible with dexketoprofen, diazepam, fentanyl, ketorolac, metamizole, phenytoin, phenobarbital and propofol.
Phenytoin and propofol mixed with 10 mg mL-1 of acetaminophen with 1 : 1 ratio showed incompatibility immediately.
Incompatibility between acetaminophen and dexketoprofen, diazepam, fentanyl, ketorolac and phenobarbital developed after an hour. The particles that were seen under microscopy in the mixture of acetaminophen with alternative medications are diazepam, dexketoprofen, diazepam, fentanyl, ketorolac and phenobarbital.
Two medications showed incompatibility with acetaminophen during the 24 hours of observation. Those are metamizole and atropine sulfate. These medications seem unable to cause incompatibility when shortly injected through the Y-site and when flushing is applied between administrations. However, this results will show incompatibility in simultaneous and continuous administration or preparation in one bag or chamber. As shown in Table 2, discoloration appeared in the sample of acetaminophen mixed with metamizole; this is usually relevant with hydrolysis oxidation with a light influence.
DISCUSSION
Acetaminophen is often administered as a secondary additive, which may interact with other medications in the Y-site connector within minutes, thus this potentially induces physical incompatibility. Acetaminophen injection formulation is a clear, colorless, free-flowing liquid under ambient light. Therefore, the changes of solution including gas, color, turbidity or particle showed the incompatibility. The precipitation due to the impact of incompatibility is a major concern in Y-site administration.
Phenytoin and propofol showed incompatibility with acetaminophen immediately. Phenytoin is a sodium salt with a very low solubility in aqueous; therefore it can induce immediate precipitation when there is contact with acid and water solution. How-ever, the lag time of induced pH precipitation is as rapid as the dwelling time of those medications in the Y-site, in which chemical changes such as degradation of the product rarely occur [10]. In addition, incompatibility of propofol and acetaminophen ensues as a result of lowering solubility of propofol and making a larger globule of the emulsion. The greater fat globule of propofol indicates flocculation associated with reduced repulsive barriers. However, a larger diameter > 5 µm may cause fatal pulmonary emboli [11].
In contrast to the abovementioned drugs, the incompatibility that develops between acetaminophen and dexketoprofen, diazepam, fentanyl, ketorolac and phenobarbital developed after an hour. The particles that were seen under microscopy in the mixture of acetaminophen with alternative medications are diazepam, dexketoprofen, diazepam, fentanyl, ketorolac and phenobarbital. Although dexketoprofen injection is freely soluble in water, it contains a basic co-solvent forming salt crystallization with a weak acid solution. Like for dexketoprofen, phenobarbital also exhibits precipitation with weak acid solution since it contains sodium salt. Diazepam itself is insoluble in a water solution; hence, it needs a co-solvent to dissolve and easily precipitates in concentration with less than a 1 : 20 ratio [12]. Fentanyl is a salt citrate with very low pH (3–4) which forms a molecule at a higher pH (6–7). A recent study proved that fentanyl is unstable in a glucose solution with higher pH [13]. Meanwhile, ketorolac is the strongest acid with tromethamine salt, which may show a salting in-salting out reaction in aqueous solution causing tromethamine precipitates.
Two medications – metamizole and atropine sulfate – showed incompatibility with acetaminophen during the 24 hours of observation. Metamizole, often known as dipyrone, is a sulfonic acid; the salt form is easily oxidized in the presence of light. Furthermore, turbidity or precipitation was also seen at 24 hours in a mixed sample of acetaminophen with atropine sulfate. Although the physical incompatibility that arises after 24 hours scarcely becomes an issue in a Y-site, we should consider the likelihood of dead stock solution from the medication in tubing. The contact between current injection and dead stock volume in the Y-site may occur after some hours of administration. This study provides a reminder that the justification of incompatibility should be interpreted carefully. The practitioners have to check and consider when, how, and what is the matter of the incompatibility.