Case report
An eighty-four-year-old woman presented to The Christie Hospital, Manchester, UK, with a 4-month history of a non-healing ulceration affecting her right anterior shin. The lesion bled occasionally, and the patient commented on the lesion catching on her clothes. Skin punch biopsy from the lesion confirmed basal cell carcinoma with mixed reticulate and infiltrative pattern, with no evidence of an atypical squamous component and no lymphovascular or perineural invasion.
On clinical examination, the lesion measured 1.6 cm × 1.3 cm and was approximately 2.56 mm thick as per high frequency skin ultrasound. The lesion did not tether underlying tissues and there was no palpable regional lymphadenopathy present. Skin condition and vascularization on the lower legs were assessed as adequate for the patient’s age, with no evidence of major atrophy or significant vascular insufficiency. The patient was otherwise fine and fit for her age. Her past medical history included gastric MALToma, left hemicolectomy for Dukes B sigmoid carcinoma, and hypertension.
She was reviewed by her surgical team and declined excision having a background of varicose veins in the past. After detailed discussion with the patient regarding possible treatment options, she agreed for superficial high-dose-rate (HDR) brachytherapy (BT) and was referred to the Brachytherapy Department at the Christie.
The patient provided written consent to take medical illustration and agreed for publishing her case report, including photographs. The patient specifically agreed for the case to be used for educational purposes. Rationale and logistics for skin BT, together with possible acute and chronic side effects from treatment were discussed in detail and the patient provided written permission for skin BT. Due to lower leg location, during consent, the patient was particularly informed of increased risk of long-term toxicity with possible non-healing skin ulceration in BT site, requiring long-term dressings and/or surgical involvement. In line with The Christie guidelines, she was also advised on skin care during and after BT, and provided with written information on skin radiotherapy, including skin care leaflet [1,2]. In the skin care advice, patients are encouraged to use any moisturizing cream as per patient wish, providing that it does not cause any contact allergy. Skin moisturizing cream should be applied during and after radiotherapy.
Brachytherapy technique
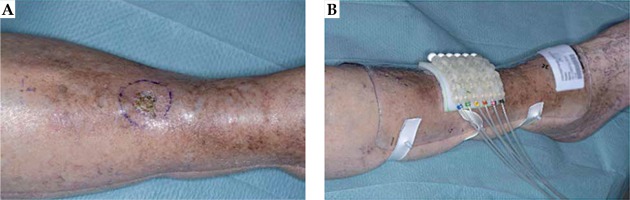
The patient was treated with superficial HDR-BT with mould. The mould was 9 mm thick and composed of 4 mm bolus and 5 mm flap as per Christie skin BT method (Figure 1). Three-dimensional computed tomography (CT)-based planning was done using CT slices 2 mm thick reconstructed at 1 mm using CT Big Bore (Philips Medical Systems B.V., The Netherlands). To ensure proper visualization, metallic markers were placed at the edge of the tumor area and in the tubes. During the planning scan, the proper positioning of the mould with flap was verified in order to avoid any misplacement or air gaps. Planning was done using Oncentra Brachytherapy version 4.5.2 (Elekta Instrument AB Stockholm, Sweden). Evaluation of the plan was performed using slice by slice visualization. The technique at the time of this treatment was to prescribe to the skin surface and cover the treatment volume with the 80% isodose line. Currently, the Christie protocol prescribes skin BT to 100% isodose in line with other BT centers. The dose was prescribed at 3 mm depth.
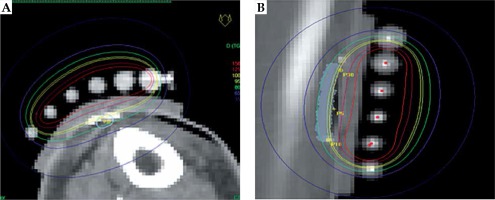
The patient received HDR-BT with a superficial mould. Due to the location of the lesion on the lower leg, the Christie standard prescribed dose of 40 Gy to 80% isodose (equivalent of 32 Gy to 100% isodose) was reduced in this case to 37.50 Gy to 80% (equivalent of 30 Gy to 100%). Further details of the Christie technique at the time of this treatment are included in Table 1. The dose was delivered in eight fractions, twice a day, with a minimum six-hour break between fractions over four consecutive days. The prescribed BT course was delivered without any problems and the patient was fully compliant. HDR-BT plan parameters are presented in Figure 2.
Table 1
Treatment plan parameters for skin HDR brachytherapy
Follow-up
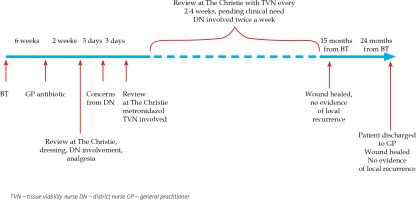
No skin reaction was noted by the treating team during or immediately after BT. Six weeks after the completion of BT, around the time of routine post-BT follow-up appointment, the general practitioner (GP) contacted the treating team to inform that the patient had developed a skin infection within the radiotherapy area and was prescribed flucloxacillin empirically 500 mg q.d.s. She was reviewed at the Christie at the end of her 2-week course of antibiotic. At that time, her anterior shin wound in the BT site appeared weepy with very fragile surrounding skin. The patient was very symptomatic from her wound and any intervention over it caused significant pain. Her wound was cleaned with saline and non-adhesive dressing (Mepilex®) was placed. No further antibiotic was felt necessary at this stage. She was also prescribed oral painkillers (paracetamol 1 g and codeine 30 mg, both p.r.n. and maximally q.d.s.). The patient was also referred to the community dressing team for further management, which could be provided more locally for the patient.
Three days after the hospital appointment, the community district nurse contacted the treating team concerned about the wound appearance, mainly black in color with odorous discharge. The patient was reviewed in the next three days at the Christie. The clinical examination revealed a large odorous wound measuring 6 cm × 4 cm with visible central necrotic masses. Wound swab confirmed significant growth of anaerobes and she was prescribed oral metronidazole 400 mg t.i.d. for 7 days. Her wound was cleaned and a non-adhesive dressing (Mepilex®) was applied. The patient continued paracetamol and codeine and reported improved analgesia. Follow-up visit after 7 days showed the lesion had improved in appearance with no odor present. At this stage, the hospital tissue viability nurse (TVN) was asked to review the patient and to advise on further management. In addition to regular wound cleaning, TVN added a desloughing wound algorithm to the patient’s management and suggested hydrogel. TVN also added consideration of compression bandaging following Doppler examination, providing that the oncologists did not have any concerns regarding local cancer progression. From that point, the patient attended the Christie every two to four weeks, pending clinical need. Each time, the patient was reviewed jointly by an oncologist and TVN. This close follow-up continued for the next 10 months. These appointments were organized in addition to twice a week involvement from the local community dressing team. At 5 months after the completion of BT, Coloplast® dressing was added to Mepilex® and at 6 months, Urgo-Clean® dressing was advised to help further with the debridement and desloughing phase of chronic wound inflammation. At the same time, she was referred to the community ulcer clinic where, after uneventful Doppler examination of the right lower leg, the patient was advised on compression stockings.
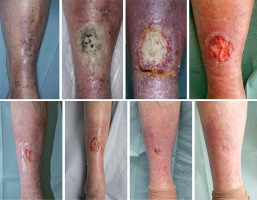
The case was also discussed with plastic surgeons and had joint review at 5 months after the completion of BT regarding possible residuum or early recurrence and to consider skin biopsy. Plastic surgeons did not feel that the picture was consistent with clinical recurrence and did not advise on skin biopsy due to very fragile skin. Plain X-ray of right tibia and fibula did not show any evidence of bone necrosis or infection at this stage. The patient continued under very close clinical follow-up with her oncology and TVN team and attended regular dressing appointments with the community dressing and ulcer clinic. Her wound eventually healed completely when reviewed 15 months after the completion of BT. Medical illustrations taken at BT planning and at follow-up are shown in Figure 3. After 2 years from the BT completion, the patient was eventually discharged back to her GP’s care being asymptomatic from her right anterior shin area, with no evidence of local or regional recurrence. Her wound has remained completely healed. Detailed time scale of events is presented in Figure 4.
Discussion
Skin lesions located on the lower legs present a significant clinical challenge. Current treatment recommendations are in favor of surgical management. Other methods of treatment involve radiotherapy [3], electrodessication, curettage, cryotherapy, and photodynamic therapy. Some guidelines do not even recommend radiotherapy in limb locations and suggest avoidance particularly in the elderly population due to risk of osteonecrosis [4,5].
In radiotherapy settings, BT offers a more favorable dose distribution to external beam radiotherapy due to rapid dose fall off and ability to spare fragile normal tissues.
Areas of poor vascularization, such as lower limbs, are subjected to constant trauma. In such locations, radiotherapy may lead to prolonged healing, radiotherapy-related ulcerations, and even necrosis, requiring surgical input. With external beam radiotherapy, particularly X-ray treatment, some authors also raise concerns regarding risk of osteonecrosis or chondronecrosis. Such risks have been particularly of concern in lesions over the anterior tibia or hand, or in ear locations, where radiation-related damaged could lead to a damage of tendons, joints, and bones, with resultant impaired hand function [6]. However, many of the patients with non-melanoma skin cancer (NMSC) on extremities are elderly and frail and/or with co-morbidities precluding surgery. In some cases, surgery requires amputation, which can be life-debilitating for the patients. Therefore, a non-invasive treatment options may be preferred. Among radiation types used in treatment of skin cancer, electron beams are common, but they prove dosimetrically challenging and are often not suitable in lesions located on curved surfaces or small skin cancers. Radiotherapy, using a BT technique, is a well-established option in such cases. In BT, the radioactive source is placed in direct proximity of the target volume, with normal tissues outside the target volume receiving a negligible radiation dose. Computer treatment planning process maximizes the dose to the target volume while minimizing unwanted dose delivery to organs at risk, such as bone or tendon. This process ensures increased chances of cure and reduced risk of late radiation-related toxicity. Therefore, it is regarded that BT offers viable radiation option in lesions over bones or cartilage, where traditional external beam radiotherapy (electrons) may be less safe [7].
In the literature, there is limited number of publications regarding the use of BT in NMSC in limb locations. Usually, literature reports gather various skin cancer locations with extremities among the others, and available outcomes are analyzed and reported together for all sites. Therefore, it is almost impossible to draw any conclusion on efficacy and toxicity of NMSC treated on extremities out of such publications. One of the biggest reported series of skin BT (radon mould technique) in NMSC comes from the Peter MacCallum Cancer Centre [8]. It included 642 patients with lesions located in upper limbs in 49% of cases and lower limbs in 17%. Gauden et al. reported on a series of 200 patients with 236 lesions treated, of which, 26 were located on extremities [9]. The treatment was delivered by HDR-BT, with Leipzig-type applicator. Total dose of 36 Gy, in 3 Gy per fraction, given daily over 2 weeks was prescribed to between 3 to 4 mm. Local control was achieved in 98% of cases. Grade 1 acute skin toxicity was reported in 168 treated lesions (71%) and grade 2, in 81 (34%). Cosmesis was assessed as good or excellent in 208 cases (88%). Late skin hypopigmentation was noted in 13 cases (5.5%). Four patients recurred locally and underwent subsequent surgery.
Svoboda et al. published a series of 137 skin lesions of various pathologies (primary skin cancers and metastases to skin from other origins) treated in 87 patients with HDR-BT. Total dose ranged from 12-50 Gy, given in 1-15 fractions prescribed at the surface of applicator. Out of 137 lesions treated, 16 lesions were located on the hands, 5 on the arms, and 13 on the lower extremities. Full response was obtained in all but four basal cell carcinomas (BCCs). The authors did not report any severe acute or late toxicity [10].
Joslin and colleagues elaborated on 20 cases treated with 45-47.5 Gy in 10 or 11 fractions [11]. Poor healing affected 3 cases, with superficial necrosis involving 3 other cases (one following injury). The authors concluded that careful consideration of dose fractionation regimes is required in lower extremities.
Many of the published articles in the field are relatively old, some presenting older techniques in radiotherapy and BT pre-computer planning. This transpires also in the references section below. Unfortunately, the information on significant radiation toxicity because of BT persists among our non-BT colleagues. It is ultimately up to us to increase our BT presence and show the wider audience not only what we do well, but also what we have learned from past experience, and how BT has improved in terms of efficacy and safety for our patients and staff. There is however a recently observed increased number of publications on the benefits of skin BT, including leg location [12,13,14,15]. Some of these publications specifically focus on the benefit of BT in the elderly population [16,17]. We sincerely hope that our readers would further contribute to this trend.
Issues suggested for Readers’ consideration (not exhaustive)
In order to start discussion, we have suggested a few questions to our Readers
Would you offer BT in this case/location or maybe external beam radiotherapy instead? If so, why?
Is fractionation adequate or should this be altered, e.g., to daily. If so, what is your proposed fractionation schedule in this case?
Could the immediate post-BT infection be a contributing factor in radiation-related toxicities?
When should we consider skin biopsy in such complex cases?
Any comments or suggestions on wound management?
Would this experience change how you counsel a patient with skin BT on leg location?
Suggested further reading
Shack RB. Management of radiation ulcers. South Med J 1982; 75: 1462-1466.
Bin Mh Busra MF, Chowdhury SR, Bin Ismail F et al. Tissue – engineered skin substitute enhances wound healing after radiation therapy. Adv Skin Wound Care 2016; 29: 120-129.
Podd TJ. Treatment of lower limb basal cell and squamous cell carcinomas with radiotherapy. Clin Oncol (R Coll Radiol) 1992; 4: 44-45.
Cox NH, Dyson P. Wound healing on the lower leg after radiotherapy or cryotherapy of Bowen’s disease and other malignant skin lesions. Br J Dermatol 1995; 133: 60-65.