Dear Editor,
We would like to present a case of a young male patient with fulminant myocarditis and multi-organ failure treated in our intensive care unit. In the early phase of the treatment, we simultaneously applied mechanical circulatory support (MCS) devices, including veno-arterial extracorporeal membrane oxygenation (ECMO). The use of short-term MCS devices in fulminant myocarditis and in other forms of severe heart failure has increased in recent years [1]. The results of some clinical trials indicate that this mode of treatment, when applied in the early phase of cardiogenic shock, yields promising final results [2, 3].
A 27-year old, previously healthy man was admitted to the Emergency Department with dyspnea, chest pain, and arterial hypotension. He had been suffering from flu-like syndrome for a week prior to admission. Upon hospital arrival, his mean arterial pressure was 50 mm Hg, and the heart rate was 120 beats per minute. Transthoracic echocardiogram (TTE) revealed severe dysfunction of both ventricles with 15% left ventricular (LV) ejection fraction (EF), 8 cm s-1 velocity-time integral of the left ventricle outflow track (LVOT VTI), and 12 mm tricuspid annular plane systolic excursion (TAPSE). Laboratory tests showed troponin above 10000 ng L-1, CK MB of 75.52 ng L-1, and ProBNP of 16497 ng L-1.
Infusion of norepinephrine with a dose of 0.3 µg kg-1 min-1 was started, and the patient was immediately transferred to the catheterization laboratory (cath lab) where coronarography revealed unaffected coronary arteries. In the cath lab, an intra-aortic balloon pump (IABP) was placed, the augmentation was set at 1 : 1, and a diagnosis of fulminant myocarditis was made. Several hours later the symptoms of cardiogenic shock intensified, so the doses of norepinephrine were escalated, and epinephrine was added.
Further hemodynamic deterioration led to the implementation of peripheral veno-arterial ECMO (Cardiohelp, Maquet, Germany) using fluoroscopy. A 23-Fr multi-stage venous cannula was inserted through the right femoral vein with the tip placed in the right atrium. A 17-Fr single-stage arterial cannula was inserted into the left common femoral artery, and a 6-Fr reperfusion cannula was introduced into the left superficial femoral artery. The flow during ECMO was set at 3.8 L min-1, and the augmentation of IABP was continued at 1 : 1. Immediately after ECMO was commenced in the cath lab, the patient was transferred to our intensive care unit.
As a result of respiratory decompensation, the patient was intubated and assisted mechanical ventilation with analgosedation was commenced. Moreover, due to symptoms of acute leg ischemia, the patient was transferred to the operating room where the dysfunctional reperfusion cannula was removed, and a 6-Fr cannula into the left popliteal artery was introduced and connected to the ECMO 17-Fr arterial cannula. Limb blood supply returned to normal. After surgery, the patient developed severe metabolic acidosis, with acute kidney injury and acute liver failure. Continuous renal replacement therapy (CRRT) with heparin anticoagulation was introduced. Prismaflex (Gambro, Sweden) was integrated into the ECMO circuit.
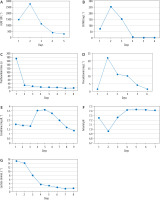
All microbiological tests were negative except for the urinary Legionella antigen test. Laboratory tests revealed highly elevated values of troponin, CK MB, ProBNP, creatinine, ASPAT, and ALAT. The main laboratory parameters from the period of ECMO conduction are presented in Figure 1. Control TTE revealed the presence of echogenic blood in LV, 8% EF LV, moderate mitral regurgitation, and TAPSE of 8 mm. There was no movement of the aortic valve leaflets with a pulseless arterial pressure curve. The echocardiographic parameters are summarized in Table 1.
TABLE 1
Echocardiographic findings during the stay in the ICU
| Day in the ICU | 1 | 2 | 3–5 | 6 | 7 | 8 | 9–10 | 11–28 |
|---|---|---|---|---|---|---|---|---|
| Mode of MCS | IABP | IABP + ECMO | Impella CP + ECMO | Impella CP | None | |||
| LV EF (%) | 15 | 8 | 15 | 30 | 35 | 35 | 30 | 30 |
| TAPSE (mm) | 12 | 8 | 13 | 23 | 20.4 | 17.4 | 17 | 18 |
| AV closure | No | Yes | No | No | No | No | No | No |
On day 2, a series of echocardiograms confirmed maintained LV distension, no movement of aortic valve leaflets, and mild-to-moderate mitral regurgitation. Several attempts at the temporary reduction of ECMO flow even to 2 L min-1 failed to open the aortic valve. In the cath lab, the IABP was upgraded to the Impella CP (Abiomed, Danvers MA) device using the same access via the right common femoral artery. The initial hemodynamic support of the extracorporeal devices was as follows: ECMO – 2.5 L min-1, Impella CP – 3 L min-1. After lowering the ECMO flow, TTE confirmed the movement of the aortic valve leaflets.
Severe hemostatic abnormalities with concomitant massive bleeding arose on day 2 and persisted for over a week. The bleeding occurred from the digestive tract, cannulation sites, tongue, pharynx, nose, and trachea. The symptoms were corrected with multiple transfusions of blood products. A sizeable hematoma of the root of the tongue was reabsorbed within the following two weeks.
On days 3 and 4 the hemodynamic status was relatively constant with the LV EF at 15%, the distention of LV became slightly reduced, and pulsatile blood flow was present. MCS was maintained the following days with an ECMO flow of approximately 2.4 L min-1, and Impella CP flow of approximately 2 L min-1. The contractility of both ventricles gradually improved, reaching an LV EF of 30% and TAPSE of over 16 mm. Improvements in kidney and liver function were also observed, and hemostatic abnormalities receded. After a reduction of the ECMO support to 1.5 L min-1, the ECMO was successfully weaned on day 8. The support of the Impella CP was maintained until day 10 and then removed at the bedside. The remaining days in the intensive care unit (ICU) were affected by severe critical illness polyneuropathy and ventilatory-associated pneumonia caused by multi-drug resistant Acinetobacter baumannii.
A tracheostomy was performed on day 18 and the patient was weaned from the mechanical ventilation the next day. Physiotherapy was intensified, yielding progressive improvements in the patient’s mobilization. The patient was finally transferred from the ICU to the cardiology unit on day 28 breathing through a tracheostomy without dyspnea and able to walk by himself.
We have described the successful combined deployment of veno-arterial ECMO and the Impella CP in fulminant myocarditis. Fulminant myocarditis is the most critical form of myocarditis usually caused by infection, drugs or the autoimmune process. Patients with fulminant myocarditis have higher rates of death and heart transplantation than patients with non-fulminant myocarditis [4]. Fulminant myocarditis can manifest itself as a cardiogenic shock with severe biventricular failure.
Several MCS methods are used when pharmacological therapy is insufficient in profound cardiogenic shock. Apart from IABP, one of the catheter-based ventricular assist devices (e.g. Tandem-Heart, Impella) as well as veno-arterial ECMO may be implemented [5]. The most commonly used device – IABP – reduces LV afterload and can passively provide up to 0.5 L min-1 of cardiac output. In our case, this mode rapidly proved to be insufficient. Due to the deterioration of the patient’s condition, we implemented veno-arterial ECMO on the same day. The most important advantage of veno-arterial ECMO is the ability to improve the blood supply to vital organs, thereby preventing the development of multi-organ failure [6, 7]. The peripherally implemented ECMO system transfers blood into the aorta in the opposite direction to the blood ejected from the LV. Consequently, and as in the case our patient, it increases the afterload of the damaged LV, which can cause aortic valve closure, intraventricular blood retention, and LV distension [8, 9]. Such a state generates excessive LV wall tension, diminishes coronary flow and increases the risk of intraventricular clotting formation. To counteract these drawbacks, temporary MCS devices are generally used – IABP or microaxial pump Impella (USA) – to unload the LV [10].
The IABP is the least invasive strategy, but its usefulness is limited [11]. In our case, we replaced the IABP with the Impella CP device. The Impella device family (Impella 2.5, Impella CP, Impella 5.0) is a group of various types of microaxial pumps which are inserted peripherally and placed through the aortic valve inside the LV. Actively pumping (up to 4 L min-1) the blood from the LV to the aorta, the Impella CP significantly reduces LV distension and wall tension. In addition, after Impella implantation, the ECMO flow can be reduced, which decreases the LV afterload. As a consequence, the aortic valve opens, and the blood flow becomes pulsatile. These features make the Impella CP an effective device in cardiogenic shock and for LV unloading [12].
The issue is more complex in biventricular heart failure, because the Impella CP should not be used as the only device in coexisting right ventricle failure. In theory, the Impella CP can be implemented alongside the Impella RP, but in practice this is challenging [13]. In the case described here, only the concomitant use of veno-arterial ECMO and the Impella CP proved to be the optimal way of treatment. The main assets of both devices were successfully applied. Veno-arterial ECMO was useful in the prevention of further multi-organ failure. In addition, reducing preload ECMO had a beneficial effect on the impaired right ventricle. The Impella CP, on the other hand, was crucial in effective LV unloading and facilitated early weaning from the ECMO. Recent meta-analyses confirmed that the implementation of LV unloading during veno-arterial ECMO is associated with decreased mortality [14, 15]. The benefits of simultaneously applying the veno-arterial ECMO and the Impella have also been reported [16, 17].
The treatment of the patient in cardiogenic shock with coexisting multi-organ failure on veno-arterial ECMO requires an interdisciplinary approach [18]. In our case, the insertion of the ECMO and the Impella implementation were performed in the cath lab. The removal of the arterial ECMO cannula and vascular intervention due to the leg ischemia were treated by a specialist in vascular surgery. A laryngologist was also repeatedly consulted regarding the hemorrhage of the tongue and massive bleeding from the pharynx.
The clinical course of our patient was affected by the consequences of multi-organ failure as well as the treatment methods implemented. The cardiogenic shock, respiratory failure, kidney injury, and liver failure required an intensive care environment. The aftermath of the patient’s treatment was multi-sited hemorrhage and severe ventilatory-associated pneumonia. Critical illness polyneuropathy was connected to the duration of analgosedation. The additional major problems specified above contributed to a prolonged stay of the patient (18 days after the Impella CP removal) in an intensive care setting.
In conclusion, the course of the treatment confirmed that concomitant usage of veno-arterial ECMO and Impella CP can be an optimal method of care for a patient suffering from fulminant myocarditis with multi-organ failure. In our case, this approach was advantageous compared to the implementation of a single MCS device. The coexistence of fulminant myocarditis with multi-organ failure involves combined extracorporeal procedures that can only be carried out in the ICU. Immediate access to specialists in interventional cardiology and vascular surgery is essential.