Approximately 5–10% of coronavirus disease-19 (COVID-19) patients need intensive care and respiratory support [1] and, among those who develop pneumonia, 14% need oxygen therapy due to severe respiratory failure, and 5% need mechanical ventilation [2]. The disease severity increases in patients with comorbidities, especially advanced age and hypertension [3].
Convalescent plasma therapy is classical adoptive immunotherapy. It has been used to treat SARS, MERS, and the 2009 H1N1 outbreak in the last two decades [4–7]. For this reason, it has been proposed as an adjunct treatment for COVID-19. It is thought that antibodies in the plasma obtained from recovered patients will reduce the virus load, thus limiting the severity of the disease [8].
In April 2020, some early reports indicated that, in PCR-confirmed COVID-19, convalescent plasma may result in a decrease in oxygen demand within three days, a decrease in CRP levels, and an improvement in chest radiography within the first week [9, 10]. However, in large-scale randomised controlled studies, administration of convalescent plasma was not associated with decreased disease progression or mortality [11, 12].
According to the “Turkish Republic Ministry of Health COVID-19 immune (convalescent) plasma supply and usage guidelines”, convalescent plasma can be added to the treatment in patients with severe or progressive disease, who are within 7 days of the onset of symptoms and:
are over 60 years of age, or
for patients between the ages of 18 and 60, have severe comorbidities (i.e., cancer, chronic obstructive lung disease, cardiovascular disease, hypertension, diabetes mellitus) or are using drugs which suppress the immune system;
the patient should also be without signs of pneumonia and before the need for intensive care [13].
Data on the efficacy of such a therapeutic approach are insufficient. This study aimed to investigate the clinical characteristics of COVID-19 patients admitted to the ICU as well as to compare outcomes between those who received convalescent plasma and those who did not. The main investigated outcomes were 28-day-mortality, length of hospital stay, and dynamics of laboratory parameters.
METHODS
This single-centre, retrospective, observational study was approved by the Tepecik Training and Research Hospital Local Ethics Committee (no: 2020/11-36, 14.09.2020). Due to the retrospective nature, the need for informed consent was waived.
All patients diagnosed with COVID-19 who were admitted to the ICU in the Tepecik Training and Research Hospital between 11 March 2020 and 31 January 2021 were screened for eligibility. Inclusion criteria:
Patients who died or were discharged within 48 hours from ICU admission were excluded from the analysis. Additionally, for the sake of homogeneity of the sample, we decided to exclude patients who did not receive favipiravir and were treated with hydroxychloroquine and azithromycin.
Clinical data were obtained from medical records. The need for non-invasive respiratory support (low-flow oxygen; non-invasive ventilation [NIV] or high-flow oxygen) and rate of invasive mechanical ventilation (IMV) were recorded [16]. Laboratory parameters on days 0, 7, and 14 were also recorded. Temporal dynamics of selected parameters were analysed in patients still in the ICU after 14 days from admission (only in the convalescent plasma group).
The counsel of an infectious disease specialist and an intensive care specialist implemented the decision to administer therapy with convalescent plasma. Convalescent plasma was given to patients according to the “Turkish Ministry of Health COVID-19 immune (convalescent) plasma supply and usage guidelines” [13]. Convalescent plasma was administered to patients twice in the amount of 200 mL, with an interval of at least 24 hours. Therapeutic apheresis centres and the Turkish Red Crescent were licensed by the Republic of Turkey Ministry of Health for convalescent plasma collection from donors. The criteria for plasma donor eligibility [13] were the following: evidence of COVID-19 documented by laboratory testing, resolution of symptoms at least 14 days prior to donation, and testing negative for HBsAg, anti-HCV, anti-HIV 1–2, and anti-syphilis antibodies [13]. Anti-SARS-CoV-2 IgG antibodies were not routinely screened in COVID-19 patients prior to convalescent plasma treatment.
Statistical analysis
Data are presented as a proportion (percentage), median (minimum–maximum), or mean ± standard deviation. Categorical variables were compared using the χ2 test. The Mann-Whitney U test was used to compare continuous variables. The Friedman test and the Wilcoxon signed-rank test were used to test the differences of paired data. A P-value of < 0.05 was considered statistically significant. Data analysis was performed using SPSS 15.0 (SPSS Inc., USA).
RESULTS
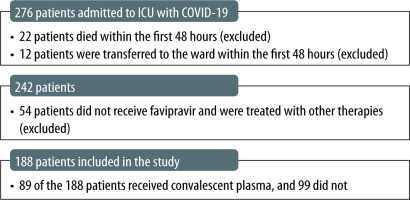
In total, 276 cases of patients with severe COVID-19 disease admitted to the ICU between 11 March 2020 and 31 January 2021 were reviewed. Of those, 34 were excluded due to ICU stay shorter than 48 hours and 54 were treated with therapies other than favipiravir and thus excluded. All in all, 188 patients were included in the analysis, 89 of whom have received convalescent plasma (Group 1) and 99 have not (Group 2). The study flowchart is presented in Figure 1.
The mean age of the patients was 67 ± 13 and there was no significant difference between the two groups (P = 0.24). The proportion of male patients was significantly higher in Group 1 [63/89 (70%) vs. 57/99 (57%), P = 0.06]. There were no statistically significant differences in comorbidities between the groups. The characteristics of the patients are presented in Table 1.
TABLE 1
Basal characteristic features of the patients
The median time from hospital admission to transfusion was 6 (5–9) days. Three patients received convalescent plasma before ICU admission (1, 2, 3 days before ICU admission, respectively). The median time from ICU admission to transfusion was 1 (0–3) day. All patients had acute respiratory distress syndrome (ARDS) at the time of transfusion. No adverse events attributed to plasma transfusion occurred within 24 hours after transfusion.
Although the mean APACHE II score was higher in Group 2, the difference was not statistically significant [15 (11–40) vs. 17 (10–39), P = 0.07]. In Group 1 more patients received steroid treatment [80/88 (89%) vs. 78/99 (78%), P = 0.03], and the rate of IMV on admission was lower [15/88 (16%) vs. 30/99 (30), P = 0.03].
While there was no difference in the overall length of hospital stay [17 (4–98) vs. 16 (3–67) days, P = 0.13], the duration of stay in the ICU was longer in Group 1 [12 (3–67) vs. 9.5 (3–74) days, P = 0.01]. 28-day mortality was 53/89 (59%) in Group 1 and 65/99 (65%) in Group 2 (P = 0.38).
There was no significant change in PaO2/FiO2 levels on days 7 and 14 in the convalescent plasma group (P = 0.10).
The temporal dynamics of laboratory parameters of 44 patients from Group 1 still in the ICU on the 14th day were analysed. Significant changes in haemoglobin and platelet levels were found on the 7th and 14th days (P < 0.001, P = 0.001, respectively) (Table 2). While there was no change in lymphocyte count in the convalescent plasma group compared to the 7th day, an increase was observed on the 14th day (P = 0.31, P = 0.01, respectively). LDH decreased over 14 days (P = 0.02). There was no significant change in CRP (P = 0.12), procalcitonin (P = 0.07), AST (P = 0.51), or ALT levels (P = 0.67) (Table 2).
TABLE 2
Oxygenation and laboratory parameters of patients receiving convalescent plasma treatment over time
DISCUSSION
We retrospectively analysed the data from patients with COVID-19 hospitalised in our ICU and found no evidence of a benefit of convalescent plasma therapy, which is in line with findings from recent randomised controlled trials. Convalescent plasma treatment, which was seen as promising in the early period of the COVID-19 pandemic, was found not to affect mortality in controlled studies that emerged later [9–12]. However, some clinical improvement within 28 days was observed in the subgroup with severe COVID-19 [11]. No significant difference in hospital stay was observed between those who received convalescent plasma therapy and those who did not, with conflicting results [17–19]. In their retrospective study, Atlanta et al. [20] noted that the time spent in the ICU and the rate of mechanical ventilation decreased with convalescent plasma therapy. Differences in lengths of stay or clinical outcomes may depend on the non-homogeneity of trials regarding the severity of disease and the length of time between symptoms and convalescent plasma transfusion. In the largest study on convalescent plasma for COVID-19 (RECOVERY), convalescent plasma was not found beneficial in terms of length of hospital stay, need for invasive mechanical ventilation, or 28-day mortality [21]. In this study, all critically ill patients were admitted to the ICU and had ARDS, with a median PaO2/FiO2 of < 100, where mortality is supposed to be very high. Convalescent immune plasma provides passive immunity [22] which is thought to be effective early after the onset of symptoms [11, 13]. It is believed that the treatment may be effective only in the first 7–10 days of infection [13] and it has been confirmed that convalescent plasma administration at the end of the course of the disease is ineffective in reducing mortality [22]. However, in the RECOVERY study, even early administration (i.e., < 4 days from illness onset) was not associated with favourable outcomes and survival [21].
Due to the retrospective nature of our study, we could not conduct a comparable analysis, because, among other reasons, we could not objectively determine the time of symptom onset. Although the delay in the delivery of the convalescent plasma may pose a problem, the first dose was administered at a median of six days after hospital admission. We did not observe any significant side effects of immune plasma in our patients, which supports data regarding the safety of immune plasma therapy [9, 23].
Although an increase in lymphocytes and a decrease in LDH were observed in patients who received convalescent plasma over time, we did not observe any significant changes in PaO2/FiO2 or CRP. In previous COVID PCR (+) patients, it has been reported that there was a decrease in oxygen demand within three days, a decrease in CRP levels, and an improvement in chest radiography in the first week [9, 10]. However, we did not observe a similar effect.
This study has some limitations. Because it is retrospective, bias cannot be ruled out. The small sample size and the lack of anti-SARS-CoV-2 IgG levels make it difficult to examine the clinical course and the data regarding the timing of administration of plasma therapy. It is thought that sufficient neutralizing antibodies must be present in the donor plasma for convalescent immune plasma treatment to be beneficial [23]. On the other hand, in the RECOVERY trial, high titre (i.e., high concentrations of neutralizing antibodies) convalescent plasma was not associated with favourable outcomes and survival [21]. The effects of neutralizing immunoglobulin antibodies in patients and donor plasma are unknown since the neutralizing antibody has not been tested.
In conclusion, in our retrospective analysis involving critical intensive care patients diagnosed with COVID-19, convalescent plasma treatment did not reduce the length of hospital stay or mortality and was associated with longer ICU stay.