Dear Editor,
Here, we present a case of rapidly progressing disseminated intravascular coagulation (DIC) following bifurcated stent graft implantation in a patient with symptomatic large abdominal aortic and iliac artery aneurysm.
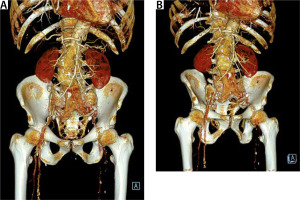
A 71-year-old patient was admitted to the vascular surgery department during duty hours with a symptomatic aneurysm of the abdominal aorta and iliac arteries, 77 mm in dia-meter, diagnosed in computed tomography. The patient was suffering from ischae-mic heart disease (condition after myocardial infarction with ST-segment elevation two months prior to admission), chronic kidney disease, generalized atherosclerosis, and hyperlipidaemia. On admission, the general condition of the patient was fairly good, with circulatory and respiratory efficiency, and moderate pain in the abdominal cavity. Due to the high arterial pressure, an intravenous infusion of vasodilators was used, which resulted in pain relief.
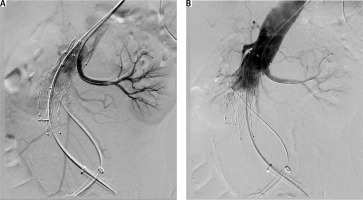
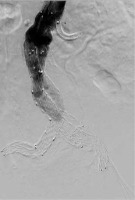
Due to the inability to discontinue antiplatelet therapy, the patient was urgently qualified for EVAR (endovascular aneurysm repair). The patient was classified as class IV according to the American Society of Anaesthesiologists (ASA) Physical Status Classification System and qualified for local anaesthesia. During the procedure, vital parameters were monitored (blood pressure measured by indirect method, SaO2, ECG). The urinary bladder was catheterized and diuresis was monitored hourly. Passive oxygen therapy through a mask was applied. Before the start of the procedure, 1 γ of paracetamol intravenously (as part of advance analgesia) and cefazolin (according to the principles of local prevention of infections in the hospital) were administered. Inguinal puncture sites were anesthetized with a solution containing a mixture of 2% lidocaine and 0.5% bupivacaine, not exceeding the maximum dose. All punctures were ultrasound-guided. Difficult aneurysm configuration was associated with extended duration of the procedure. In the first stage of the procedure, a bifurcated main body stent graft and a hidden stent into the left renal artery were implanted. In the next stages, graft limbs were embedded. The final angiography confirmed the integrity of the stent graft and no evidence of extravasation or leakage. The operator’s attention was drawn to the lack of flow in the left renal artery and a significant reduction in flow in the vessels of the right kidney. On this basis, it was concluded that intravascular coagulation may be a possible cause. For this reason, Acty-lise was administered to both renal arteries (2.5 mg each), with no resultant flow through their lumen. During the procedure, increasing anaemia of the patient was observed, requiring the transfusion of 5 units of concentrated red blood cells (RBC) and 4 units of fresh frozen plasma (FFP). In response to the characteristics of the developing hypovolemic shock, a decision was made to convert to general anaesthesia. The trachea was intubated and SIMV VC lung replacement was initiated. A central venepuncture into the right internal jugular vein was performed with ultrasound assistance, a cannula into the right radial artery was inserted, and invasive hemodynamic monitoring was implemented. The patient was heated passively (infusion of warm fluids, elimination of loss through surgical drapes). Due to the increasing hypotension, the norepinephrine infusion was started (in the flow of 0.6–1.2 mg h-1), followed by the infusion of adrenaline (in the flow of 0.05–0.1 μg kg-1 min-1), resulting in systolic arterial pressure at about 90 mm Hg. Metabolic acidosis was observed in the gasometric test performed intraoperatively (Table 1). 8.4% sodium bicarbo-nate solution was used intravenously (10 γ in total), the parameters of mechanical ventilation were modified in order to optimize the delivery of oxygen to the tissues. Empirical antibiotic therapy was extended to include metronidazole. Growing oliguria was observed followed by anuria. The re-performed angiography and ultrasound did not show any extravasation beyond the stent graft. After the end of the procedure (which lasted 5.5 hours), the patient in a very serious condition was transferred to the intensive care unit (ICU) for further treatment.
TABLE 1
Summary of laboratory results
On admission to the ICU, the patient was in profound hypovolemic shock. The inadequate circulatory system required the adrenaline and noradrenaline infusions to be continued. In addition to the centralization of the circulatory system, persistent priapism was observed. Transfusion of blood products was continued according to the results of laboratory tests, which were performed every 2–6 hours (Table 1).
The haemoglobin level was normalized, while a further decrease in the number of platelets and increasing disturbances in the coagulation system parameters were observed. Bleeding from the injection sites persisted despite the use of pressure. Based on the clinical picture, a rapidly progressive DIC syndrome was suspected. More units of FFP, cryoprecipitate, and platelets were transfused. Due to anuria, increasing metabolic acidosis and the lack of response to pharmacological treatment, continuous venous-venous haemodialysis with citrate (CVVHD CiCa) was initiated. The increasing deficiency of calcium ions was replaced. Despite intensive treatment, the coagulation system was not normalized. Due to the accompanying haemodynamic instability, the doses of vasopressors were increased. At 10 postoperative hours, slow anaemisation and increasing abdominal circumference were observed. The patient was surgically consulted. Bedside ultrasound examination of the abdominal cavity revealed impaired vascular flow in the right kidney and its absence in the left kidney, a clotted aneurysm bulb and preserved flow in the stent graft limbs. The patient was not qualified for surgical treatment. Successive FFP and RBC units were transfused without success. Despite aggressive treatment in the intensive care unit, the patient’s condition deteriorated rapidly – increasing multi-organ failure was observed. Death took place on the first postoperative day, with a full-blown plasma-platelet defect. Due to the irreversible cause of the disease, cardiopulmonary resuscitation was not undertaken.
DIC is characterized by systemic activation of the coagulation system, leading to the formation and deposition of fibrin in the vascular bed of internal organs, its coagulation and, consequently, the development of the multiple organ dysfunction syndrome (MODS) [1, 2]. The consumption of clotting factors and the reduction in the number of platelets, which are a manifestation of DIC syndrome, lead to life-threatening bleeding [3], which was observed in the presented case. The prognosis for DIC varies depending on the severity of the coagulopathy and its underlying cause. A key element in the development of intravascular coagulation is the imbalance between procoagulation processes, inhibitory systems and fibrinolysis. Due to the risk of reduced fibrinolysis and secondary progression of thrombosis, the use of antifibrinolytics is not usually recommended. For this reason, the infusion of unfractionated heparin was not used in the present case. DIC diagnosis is based on meeting the criteria included in the scales approved by the International Society on Thrombosis and Haemostasis (ISTS). The most popular and useful tool used by clinicians is the 5-step diagnostic algorithm for the diagnosis of acute disseminated intravascular coagulation [4] (Table 2).
TABLE 2
Scoring algorithm for the diagnosis of acute disseminated intravascular coagulation (DIC)
In the discussed case, the patient obtained 7 points in each of the control measurements. Despite aggressive treatment, the score did not change. The presented surgical technique (EVAR) initiated the process of coagulation and simultaneous fibrinolysis by turning off the large volume of blood trapped in the space of the aneurysm bulb. The observed intraoperative bleeding resulted from increasing coagulation disorders (rapid increase in blood loss from injection sites with the accompanying exhaustion of the body’s adaptive mechanisms). Additionally, within the aneurysm bulb, there was an inflammatory process [5, 6] that could be responsible for the distribution component of shock. Rapidly progressive DIC is basically an irreversible process that does not respond to aggressive treatment and is associated (as in the presented case) with therapeutic failure.