Introduction
The global prevalence of psoriasis is estimated at 1% to 3%, of which 85–90% is plaque psoriasis, chronic, inflammatory, immune-mediated dermatosis characterized by well demarcated erythematous-papular lesions covered with scales [1]. Histologically, there is hyperproliferation and incomplete differentiation of keratinocytes, acanthosis, retention of cell nuclei within stratum corneum, and loss of the granular layer. The epidermis has a dense infiltrate of T lymphocytes and dendritic cells (DC) [2]. In psoriasis, there is an immunologically induced hyperproliferation and functional stimulation of keratinocytes, which is mediated by numerous cytokines released by T lymphocytes, including interleukin (IL) 1 (IL-1), IL-6, IL-8, IL-12, IL-17, IL-18, IL-22, IL-23, tumour necrosis factor (TNF), interferon (IFN), and transforming growth factor-β (TGF-β). Proinflammatory cytokines are also secreted by epidermal and dermal cells, including keratinocytes, DC, endothelial cells and fibroblasts [3]. A characteristic feature is the appearance of psoriatic lesions in specific places, as well as recurrences of the disease in the same location after discontinuation of treatment. Despite the clinical resolution of the lesions, within the dermis and epidermis, differences in the populations of cells of the immune system and at the molecular level, constituting local memory, are still observed [4]. Cells responsible for local memory include primarily tissue resident memory T cells (TRM), but regulatory T lymphocytes (Treg), DC, and Langerhans cells (LC) also seem to play an important role in this process. The types of cells, their interactions, and mechanisms that may be involved in molecular scar formation and maintaining the local memory of disease, as well as the effect of various therapies on their levels, are discussed below.
Types of cells forming local memory in the skin
Tissue resident memory T cells
Tissue resident memory T cells are a subset of non-circulating memory T cells. They arise as a result of the activation of naive T cells by antigen-presenting cells. Precursor cells evolve into few subsets of memory T cells, including central memory T cells (TCM), effector memory T cells (TEM), and tissue resident memory T cells (TRM). TEM predominate in peripheral non-lymphoid tissues, while TCM are localized in secondary lymphoid organs [5]. TRM cells are phenotypically distinct because they express molecules that prevent tissue egress, in particular the CD69 antigen responsible for TRM deposition by prevention of their recirculation through suppression of the sphingosine-1-phosphate receptor (S1P1) or the CD103-E-cadherin adhesion receptor, determining the localization of TRM in the epithelial tissue. TRM also express the CD49a molecule, which links collagen IV found in the basal layer [6]. In addition, TRM have receptors for chemokines such as CCR4, CCR8, CCR10, CXCR3, and CXCR6, which are considered as salient molecules for directing or retaining TRM in the skin [7]. The most important task of TRM is the protective function against infections; however, they are also involved in the pathogenesis of many dermatological diseases, including vitiligo, lymphomas, drug eruptions, and psoriasis.
T lymphocytes infiltrating psoriatic lesions play a crucial effector role, stimulating the development and maintenance of the disease [8]. Gilhar et al. transplanted psoriatic skin into the severe combined immunodeficient (SCID) mice, which were then injected with autologous T cells. Maintenance of skin pathology within a transplanted psoriatic skin lesion was not only dependent on T-cell, but skin-originated T cells were more effective in maintaining pathology compared to blood-originated T cells from psoriatic patients [9]. Another study revealed that immunodeficient mice can evolve psoriatic plaques after transplantation of healthy-looking skin from mice with psoriasis [10].
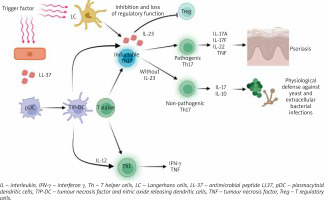
In the skin of patients suffering from psoriasis, TRM consists of CD8 and CD4 fractions, which increase the expression of IL-17A, IL-22, and IFN-γ – inflammatory cytokines, which are of key importance in the pathogenesis of plaque psoriasis [7]. TRM can be activated upon recognition of various antigens or cytokines/chemokines, leading to a characteristic relapse after treatment. Autoantigens include but are not limited to neolipid antigens, antimicrobial peptide LL37, and disintegrin and metalloproteinase with thrombospondin motif-like protein 5 (ADAMTSL5) produced by melanocytes [2, 6], PLAG4D from mast cells and keratinocytes, and keratin 17 from keratinocytes [7]. The antimicrobial peptide LL-37, also derived from damaged keratinocytes, binds to its own DNA/RNA thus activating plasmacytoid dendritic cells (pDC) and TNF and nitric oxide releasing dendritic cells (TIP-DC), leading to the formation of key inflammatory cytokines IL-23 and TNF-α. In psoriasis, effector T cells producing IL-17A (Th17 and Tc17) are dependent on IL-23 for survival [11]. IL-23 also inhibits the function of Treg, leading to loss of their regulatory function. Activated DC produce IL-12, which stimulates Th1/Tc1 differentiation; these cells produce IFN-γ and TNF-α (Figure 1). There is also the activation of myeloid dendritic cells (mDC) migrating to the lymph nodes where they are taking part in T-cell differentiation and activation. Activated T cells migrate to affected skin through adhesion molecules on vascular endothelial cells, releasing effector molecules such as IL-17, IL-22, and IFN-γ, thereby affecting keratinocytes [2]. Keratinocytes can produce cytokines and chemokines to attract more cells such as neutrophils and macrophages, as well as produce vascular endothelial growth factor, which promotes endothelial cell proliferation and angiogenesis, thus creating highly vascularized psoriatic plaques [2].
In psoriasis, CD8+CD49a+CD103+TRM occupying the epidermis produce IFN-g and activate their cytotoxic properties after exposure to IL-15, while CD8+CD49a-CD103+TRM more frequently produce IL-17A [12]. CD4+CD69+CD103+TRM preferentially inhabit the dermis, being close to blood vessels. IL-22-producing CD4+ and IL-17A-producing CD8+ cells also remain in the epidermis of the healed lesion, possibly determining recurrence of the disease; furthermore, it appears that the percentage of CD8+CD103+ cells capable of generating IL-17A in previously unaffected skin of a patient with psoriasis increases with the duration of the disease [13, 14]. An important and interesting issue requiring further research is whether early intensive therapy can alter or modulate pathogenic TRM and affect the “molecular scar” remaining in patients’ healed skin.
Regulatory T lymphocytes
Regulatory T lymphocytes (Treg) are a subpopulation of lymphocytes capable of influencing immune cells by producing inhibitory cytokines (IL-10, IL-35, galectin-1, TGF-β) or by direct cytotoxic effect (perforin and granzyme B). Treg are involved in the immune homeostasis, as well as in maintaining tolerance and preventing autoimmune diseases [15]. During immunological homeostasis, CD4+CD25+high Forkhead box P3+ (Foxp3+) Treg maintain self-tolerance by controlling the activation of effector T cells [16]. Foxp3 is a more specific marker of Treg than CD4 and CD25 and is crucial for their evolution and activity [15]. Moreover, Treg inhibit the pro-inflammatory activity of macrophages, which are the main effector immune cells in TNF-dependent psoriasis. In the course of psoriasis, the suppressive function of Treg cells is impaired, which leads to a change in the Th17/Treg balance. It seems that it is the dysregulation of the functioning of Treg that may be important in the pathogenesis of plaque psoriasis (Figure 1) [15].
There are some discrepancies in the results of studies regarding the infiltration of Treg into the affected skin. Zhang et al. showed an increase in the number of Foxp3+Treg both in the peripheral circulation and in psoriatic skin, which positively correlated with the severity of the disease [17]. Keijsers et al., comparing samples of asymptomatic skin, lesion area, lesion edge, and centre of the psoriatic plaque, observed a relevant increase in the number of CD3+, CD4+, and Foxp3+ Treg cells from the asymptomatic skin to the centre of the psoriatic lesion [18]. While Kotb et al. observed a lower percentage of Treg cells in patients with plaque psoriasis compared to the level in healthy people [19]. Conversely, Yun et al. noticed a decrease in the number of Foxp3+Treg in lesioned skin compared to normal skin in patients with exacerbation of psoriasis, but an increase in those with chronic disease, regardless of the severity of the disease [20]. Fujimura et al. revealed more CD3+, CD4+, and CD25+Foxp3+Treg in the epidermis with psoriasis than in the dermis [21]. On the other hand, Bovenschen et al., found a more abundant CD4+CD25+Foxp3+Treg infiltration in the dermis than in the epidermis in psoriatic patients [22]. The discrepancies in the studies cited above indicate that the pathogenesis of plaque psoriasis may be influenced not by the number but by the dysfunction of Treg cells. There are several studies describing changes in Treg cell density in the skin of patients after successful treatment of psoriasis. Kotb et al. showed an increase in Treg density during UVB treatment or topical vitamin D and betamethasone [19], contrary to Keijsers et al., who reported a reducing the number of Treg cells in psoriatic patients treated with topical vitamin D and betamethasone [23]. In the case of monoclonal antibody therapy (anti-IL17A, anti-IL-23) in a mouse model, an increase in the number of Foxp3+Treg cells in previously changed skin was observed [24].
The role of Treg cells and the effect of therapy on their levels remains unclear and requires further research; however, it seems that long-term remission in psoriasis may be related to the normalization of Treg cell function, enabling the achievement of a balance between pathogenic and effector memory cells [15].
Dendritic cells, Langerhans cells
The skin contains numerous dendritic cells which include i.a. dermal dendritic cells (dDC), plasmacytoid dendritic cells (pDC), and Langerhans cells (LC). Dendritic cells DC and LC are innate immune cells, which are specialized antigen-presenting cells, inducing a T-cell immune response and maintaining tissue tolerance by promoting T-cell development, differentiation, and function. DC play a significant role in establishing and affecting the function of memory T cells in the skin, and abnormal activation of T cells due to DC dysregulation has implications for plaque psoriasis (Figure 1) [4]. Different types of DC are involved in the pathogenesis of psoriasis, and their total number is significantly increased compared to the skin of healthy individuals [4]. Injured keratinocytes release nucleic acids, antimicrobial peptides that bind through the toll-like receptor (TLR) in pDC, secreting large amounts of INF-α, promoting dDC maturation and monocyte differentiation into inflammatory DC. In active lesions, dDC have increased expression of IL-12/IL-23p40 [25] and produce many pro-inflammatory cytokines (IL-1β, IL-12, IL-23, TNF-α) [26], which activate differentiation naive T cells in Th1, Th17, and Th22. dDC have an increased ability to recruit neutrophils, keratinocytes, and T lymphocytes. Interestingly, DC, as well as being involved in the initiation of inflammation, are also important in sustaining psoriatic inflammation by maintaining the ability to produce IL-23 and TNF-α [26, 27] despite clinical resolution of skin lesions and effective therapy. Together with TRM, these cells seem to remain in the skin, causing relapses of the disease.
LC have the ability to produce IL-23 after stimulation of various types of TLR, which, combined with reduced motility in the affected skin, indicates that LC can stimulate epidermal T lymphocytes [4]. One study showed that epidermal LC present in clinically healed skin share a similar location with epidermal T cells and preserve abnormal profiles of gene expression [28]. The authors observed an increase in the expression of IL-23 and IL-15 by LC after anti-TNF treatment, which play a role in the proliferation of T lymphocytes and the generation of IL-17-producing T lymphocytes [28]. Despite achieving clinically clear skin, antigen-presenting cells appear to be on standby to express pro-inflammatory genes. The ability to cross-communicate with T cells and keratinocytes suggests that LCs play a role in T-cell reactivation and relapse. The concept of innate immune memory exists, but whether DC and LC can be considered as innate memory cells remains to be clarified [4].
The effect of treatment on the molecular scar in plaque psoriasis
Phototherapy
In the treatment of plaque psoriasis, phototherapy is most often used with narrow band UVB (nbUVB) and UVA 320-400 nm radiation. Yones et al. conducted a study comparing the effectiveness of 2 well-known therapies: PUVA and nbUVB, showing that PUVA therapy is significantly more effective and gives longer remissions than nbUVB [29]. It may result from the normalization of the expression of keratin 16, filaggrin and involucrin, as well as the suppression and reduction of CD4+ and CD8+ T lymphocytes infiltrating the epidermis and dermis [30]. Phototherapy induces apoptosis of keratinocytes and has an immunosuppressive effect. During phototherapy, the activity of anti-inflammatory cytokines (IL-4, IL-10) increases and the activity of pro-inflammatory cytokines (IL-8, IL-17, IL-22, IL-23, IFN-γ, TNF-α) decreases. Moreover, the induction of Treg and the reduction of LC levels are also observed [2]. A study assessing TRM in psoriatic plaques before nbUVB phototherapy and in healed skin after treatment, revealed that part of the TRM was found to be Th17 dominant and some were Th1 dominant. Faster disease relapses after discontinuation of therapy were found in patients with IL-17A expression (Th17 dominant) compared to TRM Th1-dominant patients [31]. In another study, the ratio of CD8+CD103+TRM producing IL-17A to those producing IFN-γ increased with the duration of the disease in non-lesional psoriatic skin [13]. The above results suggest that early implementation of treatment may be associated with a reduced risk of disease relapse. Johnson-Huang et al. showed in their study that nbUVB leads to inhibition of the IL-17/IL-23 axis. After 6 weeks of therapy, a reduction in the number of mDC and suppression of the INOS, IL-20, IL-23p19, and IL-12/IL-23p40 genes were observed. The expression of IFNG, IL-17, IL-22, and BD4 genes was also reduced, and the production of IFN-γ by 85%, and IL-17 (45%) and IL-22 (89%) was inhibited [32]. This proves that UVB-phototherapy affects the same transmission paths as modern anti-psoriasis therapies.
Biologic treatment
The accelerated TNF-α/IL-23/IL-17 axis is the main pathomechanism of plaque psoriasis, which explains the high effectiveness of biological therapies [33].
Anti-TNF-a
TNF-α inhibitors have been used in the treatment of psoriasis for over 20 years, but despite their high effectiveness, skin lesions recur quickly after treatment. Cheuk et al. assessed the TRM in the skin after resolution of psoriatic lesions under the influence of nbUVB and long-lasting biological treatment (anti-TNF-α or anti-IL-12/23). Although both nbUVB and inhibition of TNF-α or IL-12/23 greatly decreased the quantity of T cells in epidermis, the observed increase in CD8+T cells expressing the CD49a marker associated with TRM cells (5-fold for nbUVB and 16-fold for anti-TNF-α or anti-IL-12/23 compared to normal skin) implied expansion or selective retention of pathogenic TRM in resolved lesions. RNA expression profiles implied a less active TRM phenotype in skin treated with biologics. T cells expressing IL-17 and IL-22 were present in both active lesions and skin treated with nbUVB and biologics. The stable TRM population rapidly produces IL-17 or IL-22 upon restimulation, even after 6 years of biologic treatment, indicating that these cells are highly pathogenic and some of T cells located in the epidermis turn into long-living TRM [12]. In another study, the authors observed stabilization of keratinocyte proliferation and decreased expression of Th-17-related genes, i.e. IL-17, CCL20, and DEFB4, after just 1 week of anti-TNF-α treatment. A reduction in the expression of DC inflammatory products (INOS, TNF, IL-20, IL23p40) was also found. With the resolution of psoriatic plaques, a decrease in the number of macrophages [34] and a complete depletion of dendritic cells and CD3+ T lymphocytes was observed [35]. Of interest was the lack of complete depletion of CD8+T cells (64% reduction). This shows that the inflammation is not completely suppressed after the anti-TNF-α treatment, despite macroscopic resolution of the lesions. In the skin of healed patients, a molecular scar is observed, defined as the “genomic profile of residual disease” containing 248 gene products that do not normalize after treatment [35]. Matos et al. in patients treated with etanercept or UVB phototherapy showed a higher quantity of T cells in “cured” skin compared to healthy controls. Before treatment, various T-cell clones were observed in the psoriatic plaques, but only 7% of the T-cell clones remained in the cured skin lesions. After the triggering factor, these cells begin to overproduce IL-17A [2, 36].
Anti-IL-17
Inhibition of the Th-17/IL-17 pathway seems to be of key importance in the treatment of plaque psoriasis [7]. In their study, Krueger et al. found that during treatment with ixekizumab there is a reduction in keratinocyte hyperproliferation and infiltration from CD3+T lymphocytes, DC, and AMP (LL-37, S100A7, S100A8, BD2) [37]. They also observed a decrease in gene expression, e.g. IL-17A, IL-17F, IL-19, IL-22, IL-23p19, LCN, KRT16, CXCL8, and CXCL1. Interestingly, after 2 weeks of ixekizumab therapy, it was found that as many as 1200 psoriasis genes change expression, of which 600 were normalized, while etanercept therapy normalized the expression of only 100 genes [37]. Rapid improvement with ixekizumab is associated with suppression of the influence of IL-17 on keratinocytes but does not appear to affect T-cell infiltrates in the skin. In a study of patients treated for 12 weeks with secukinumab, Reich et al. observed that after 2 weeks of therapy there was a decrease in the number of neutrophils, a reduction in the expression of IL-17A and IL-17F mRNA, but no effect on TNF mRNA. In the case of the number of DC and T lymphocytes, a much slower decrease was observed. There was no decrease in mast cell counts over 12 weeks of treatment. Surprisingly, patients treated with low doses of secukinumab showed a renewed increase in the number of neutrophils after the 12th week of treatment, and a higher number of neutrophils was associated with a shorter period of clinical remission after treatment discontinuation [38]. It is the effect on neutrophils that is responsible for the rapid therapeutic effect, while the long-term effect of therapy as well as the length of remission are associated with a decrease in T lymphocytes and DC. No DC are found in the cured skin, but LC capable of producing IL-23 are still present [28].
Anti-IL-12/IL-23
Both IL-12 and IL-23 have a relevant impact on the differentiation of T lymphocytes. Brodmerkel et al., in their study, observed a decrease in the expression of genes related to the transmission pathway of IL-1, IL-17, IL-22, IFN-γ, and TNF-α during 12 weeks of treatment with ustekinumab. Interestingly, the expression of 18% of genes after treatment did not return to the baseline level, compared to 23% in the case of etanercept [39]. It seems that this “smaller” molecular scar is responsible for the greater likelihood of a longer clinical remission after ustekinumab therapy (lower expression of IL-17).
Anti-IL-23
IL-23 antagonists appear to reduce the T-cell fraction more effectively than IL-17 antagonists. This is reflected in the length of remission of the disease after biological treatment with specific methods. Although drugs directed against IL-17 give a faster clinical response to treatment, IL-23 antagonists give the longest remissions of the disease. Long-term remission in the case of the use of IL-23p19 antagonists in the psoriasis treatment was demonstrated for many months after discontinuation of therapy, even after the drug was completely washed away [40]. Appropriately early inclusion of anti-IL-23 treatment resulted in a higher rate of sustained remission compared to patients with long-term disease [41]. Flora et al., due to the many pathophysiological similarities between guttate psoriasis and plaque psoriasis, reviewed cases of patients with guttate psoriasis treated with risankizumab. In the case of guttate psoriasis, usually about 25–39% of patients develop chronic plaque psoriasis; however, after 3 doses of risankizumab and discontinuation of treatment, the authors did not document a recurrence of the disease in up to 24 months of observation in any of the patients [42]. IL-23 plays an important role in the survival of TRM in the skin of patients with psoriasis, and early IL-23 antagonism may diminish the formation and survival of TRM, which later form a “molecular scar”. Similar results were observed with ustekinumab, secukinumab, ixekizumab, and guselkumab therapies [43, 44]. In a study comparing risankizumab with ustekinumab, it was found that both drugs reduced the thickness of the epidermis, reduced the expression of proteins: CD3, CD11c, K16, Ki67, S100A7, and β-defensin-2 after 4-week treatment. In the case of both drugs, the expression of genes related to the IL-17/IL-23 transmission pathway was reduced, including IL-17A, IL-17C, IL-17F, IL-22, IL-23A, S100A8, and S100A9. However, risankizumab turned out to be more potent than ustekinumab in reducing the expression of disease-related genes [45]. Sofen et al. proved that during a 12-week therapy with guselkumab there is a significant thinning of the epidermis, and a decrease in the expression of keratin 16 and the number of CD3+ and CD11c+ cells. Lower expression of KRT16, S100A7, LCN2, CXCL1, CXCL8, and IL-17A genes was also observed. Moreover, normalization to baseline values of more than 70% of genes associated with psoriasis pathogenesis has been demonstrated [46]. Mehta et al. compared the efficacy of guselkumab and secukinumab therapy, finding that both treatments decreased the incidence of inflammatory T cells, monocytes, inflammatory DC, and CD4+CD49a-CD103-T cells. Guselkumab reduced the number of memory T cells while preserving Treg, and contrary to secukinumab, none of the drugs modified the incidence of IL-17A+IL-17F+/- CD4+ or CD8+ T cells [47].
Conventional therapies
There is still lack of reports of the “molecular scar” after conventional systemic and topical therapies. Owczarczyk-Saczonek et al. evaluated the localization and amount of TRM markers in skin lesions for 3 therapies (methotrexate, anti-IL-17, and anti-TNF). The fastest decrease in the expression of TRM markers was noticed during anti-IL-17 therapy in the fourth week of treatment, while in the case of methotrexate (MTX) and anti-TNF, the response was significant at the 12th week of therapy. The number of markers decreased mainly in the dermis but not in the epidermis [48]. Another study showed that psoriatic TRM persisted in resolved plaques even for months after successful treatment with methotrexate. After an 8-week MTX therapy, reduction in the inflammatory infiltration was observed, but CD8+ T cells with CD69 expression were still present in the healed skin lesions [49]. Kurihara et al. studied 2 groups of patients, the first treated only with topical therapy and the second treated with cyclosporine, a phosphodiesterase 4 inhibitor, or biologics. The frequency of CD8+CD103+IL-17A+ and CD4+CD103+IL-17A+ TRM was higher in the systemic therapy group, while CD8+TRM revealed a higher frequency than CD4+TRM. There was no relationship between CD8+CD103+IL-17A+ or CD4+CD103+IL-17A+TRM and the severity of primary psoriatic lesions [50]. In one of our studies, we analysed the correlation between the expression of TRM markers and the duration of the disease, showing a clear dependence between these variables. The longer the course of the disease, the greater the expression of TRM markers [14]. Similarly, to Kurihara, we found no correlation between the expression of the analysed markers and the intensity of skin lesions [14, 50].
The question that arises after analysing the above studies is whether systemic drugs and monoclonal antibodies penetrate the epidermis, where TRM are still present in the healed skin. Clark et al. assessed the effect of anti-CD52 treatment on T lymphocytes circulating in the blood and skin. Low doses of the drug were found to be effective in treating patients with refractory L-CTCL but not MF. This was due to the fact that the drug removed T lymphocytes circulating in the blood but did not eliminate T lymphocytes residing in the skin [51]. Similarly, in the case of TRM in psoriasis, systemic drugs can be highly effective in eradicating T lymphocytes in the circulation and in the dermis, leaving TRM in the epidermis [48].
Topical treatment
Kurihara et al. studied the result of topical treatments on TRM in the skin of psoriatic patients. Betamethasone dipropionate, calcipotriol, and calcipotriol/betamethasone dipropionate (Cal/BD) ointments were all shown to reduce overall CD8+CD103+TRM counts, but it was the combined preparation that reduced TRM the most. Interestingly CD8+CD103+TRM persisted near the basal membrane zone of the epidermis, being resistant to all tested topical therapies [52]. Another study assessed TRM markers in psoriatic lesions before and after 12 weeks of therapy with Cal/BD. Most markers showed a statistically significant reduction in immunofluorescence at week 12 (CD4, CD8, CD69, CD103, CXCR6, IL-17A, and IL-22) in the epidermal layer. Changes in the expression of TRM markers in the dermis were observed in the case of CD4 and IL-22, which proves that topical therapy does not substantially affect TRM in the dermal layer [53]. Perhaps it is the combination of systemic therapy with topical eradication of TRM at the epidermal level that may be an encouraging therapeutic strategy to avoid psoriasis flare-ups in previously affected skin areas.
Conclusions
Molecular scarring remains in the skin of patients despite the most modern methods of psoriasis treatment. TRM appear to provide a biological elucidation for the clinical observation that psoriatic plaques occur repeatedly in the same anatomical locations throughout the course of the disease. What is more, in “cured” psoriatic lesions there are over 250 genes whose expression does not return to the state of healthy skin [2]. Particular attention and further research are also required for LC, DC, and Treg affecting the activation of dormant TRM and involved in the formation of the “molecular scar”. The impact of treatment on TRM remains a difficult and not fully explored issue in dermatology; however, based on current scientific reports, it seems that several factors influence the proper control of the disease and reduction of TRM, including early initiation of treatment, the type of therapy used, as well as its duration. It seems that the use of combined therapies – general and topical treatment – may work more effectively on the number of TRM in the skin of psoriatic patients, giving longer and more durable remissions of the disease.