Purpose
The use of computed tomography (CT) in delineation of organs at risk (OARs) and target volumes is well-known. Vaginal cuff brachytherapy (VCB) is adjuvant treatment delivered after hysterectomy in cervical and endometrial cancer patients, alone or combined with external beam irradiation [1]. The primary purpose of the present study was to prospectively investigate the dosimetric consequences of administering rectal enemas prior to VCB. The secondary objective was to analyze the dose distribution in OARs, using standard planning with multiple points optimization 5 mm from the applicator surface such as 2D plans. The last goal was to record the presence of air gaps (AGs), which can potentially influence the treatment outcome.
Material and methods
In 2019, 75 consecutive patients with indications for post-operative VCB for endometrial or cervical cancer were recruited. Sixty-two patients with endometrial cancer and 13 patients with cervical cancer were treated. In 35 cases, VCB was the only adjuvant treatment. In 40 patients, post-operative treatment was composed of external beam irradiation and brachytherapy. All patients received post-operative high-dose-rate (HDR) VCB in an outpatient setting. Phosphate enema was performed in 38 patients, and 37 were not specially prepared before the applicator insertion. Patients who had an enema before the VCB were selected randomly. The patients were asked to empty their bladder before the procedure. Single-channel vaginal cylinders of the largest diameter (2-3.5 cm) that could fit comfortably inside the vagina vault were used. The applicators were placed while asking the patients to relax and breathe, because it is essential to use the largest possible cylinders for good coverage of vaginal mucosal tissue. The following cylinder diameters were used for the procedure: 2.0 cm (2 patients), 2.5 cm (32 patients), 3.0 cm (40 patients), and 3.5 cm (1 patient).
Pelvic CT scans were carried out in the supine position, with 3 mm slice thickness and no gap between slices. CT images were transferred to a 3D treatment planning system (Oncentra Brachy v.4.5.3, Elekta, Sweden). Organs at risk were contoured by the radiation oncologist on the consecutive CT slices according to recommendations [2]. We assessed the dose in rectum and sigmoid as the one-organ rectosigmoid. The outer bladder wall and the rectosigmoid from 1 cm over the cylinder tip to the anus were delineated. An active length of 2.75 cm (11 activated dwell source positions) was used and optimized to deliver a fraction dose of 7 Gy at 5 mm depth from the applicator surface in lateral and cranial directions. Patients were planned in accordance with the GEC-ESTRO handbook of brachytherapy, as presented in Figure 1. For a purpose of the study, we analyzed measurements for one 7 Gy fraction. Dose-volume histograms (DVHs) were generated. Brachytherapy (BT) was given to all patients using an iridium-192 (192Ir) source, with an initial nominal specific activity of 10 Ci using the MicroSelectron HDR afterloading system (Elekta). The protocol and consent procedure were approved by the local medical authority.
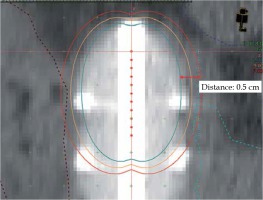
Fig. 1
Doses distribution around vaginal cylinder applicator with multiple points used for optimization
Brown – rectosigmoid, Dark blue – intestine, Light blue – bladder. Blue 259 circles are points used for optimization, Distance – 0.5 cm
For the purpose of this analysis, all plans were created according to the GEC-ESTRO recommendations without dose corrections – standard plan. In cases of very high doses in OARs or AGs over 5 mm in irradiated areas, we corrected the treatment plans individually for each patient. Decision was made by a physician based on the treatment history of patient. All air gaps were recorded in the whole length of vagina.
Statistical analysis
Descriptive statistics were used to describe and analyze a specific dataset, providing short summaries of the sample and measures of central tendency (mean, median, and mode), and measures of variability (standard deviation, variance, minimum and maximum values). The sets and distribution of our data were analyzed, and histograms of all variables were plotted. Distributions of all variables, except for the bladder volume, were considered as a normal distribution. The comparative analysis was performed using Student’s t-test (enema vs. rectal dose). Then, correlation analysis (non-parametric correlation, Spearman’s test) and analysis of variance (ANOVA) were used for bladder volume vs. bladder doses. The whole group (with and without enemas) was divided into three equal sets according to the variable determining the volume of bladder.
Results
The median age of patients was 62 years (range, 35-85 years). Data derived from DVHs for the whole group (n = 75) are presented in Table 1.
Table 1
Doses given for organs at risk for the whole group of patients (n = 75)
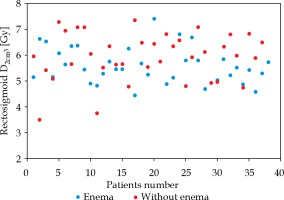
Enema was performed in 38 patients. In 37 cases, CT was completed without any special bowel preparation for the procedure. Enema before cylinder insertion did not influence rectosigmoid Dmax (p = 0.38) and D2cm3 (the minimum dose to the highest treated 2 cm3, p = 0.056) (Figure 2). Data showing mean doses for rectosigmoid with and without enema are given in Table 2.
Table 2
Mean doses to rectosigmoid for patients with enema vs. without enema
| Enema (n = 38) | Without enema (n = 37) | |
|---|---|---|
| Rectosigmoid D2cm3 [Gy] | 5.6 (SD 0.7) | 5.9 (SD 0.9) |
| Rectosigmoid Dmax [%] | 130.0 (SD 30.3) | 135.8 (SD 25.6) |
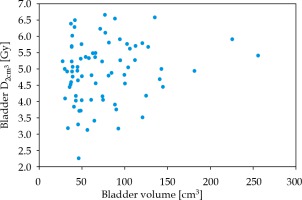
The bladder volume in the volume range of 27-256 cm3 was not associated with an increase in Dmax (p = 0.77) or D2cm3 (p = 0.181) in Student’s t-test. Bladder D2cm3 vs. bladder volume are presented in Figure 3. In the second analysis, bladder volumes were divided into three equally numerous groups: < 46 cm3, 46-84 cm3, and > 84 cm3, for a comparison. There were no bladder volume effect on bladder doses noted, D2cm3 (p = 0.597) and Dmax (p = 0.229).
The volume of organs at risk covered by the prescribed dose V100 was assessed. The median bladder V100 was 0.1 cm3 (range, 0-1.4 cm3). Rectosigmoid median V100 was 0.5 cm3 (range, 0-2.7 cm3). There were 22 patients with V100 bigger than 1 cm3 and 6 patients with V100 bigger than 2 cm3 in rectosigmoid. These 6 patients with V100 ≥ 2 cm3 had a D2cm3 doses in the range of 7.1-7.4 Gy and Dmax in the range from 9.7 Gy (139%) to 19.7 Gy (282%).
There were 62 (83%) patients with air gaps around the cylinder in the whole group. In 15 (24%) cases, AGs were located at the apex (upper 3 cm of cylinder including the top of the vault) and in 47 (75%) patients, AGs were situated in the lower part of vagina. The AGs depth was smaller than 3 mm in 37 (59%) cases and bigger than 3 mm in 25 (40%) patients. The median thickness of AGs was 3 mm (range, 2-6.5 mm). There were 5 (8%) cases with AGs bigger than 5 mm, one in the apex of vagina, and 4 within 46-57 mm below the apex. When 7 Gy was delivered at 5 mm depth from the applicator surface, the dose on vaginal mucosa was about 12.4 Gy. In case of an AG appearance with 3 mm depth, the dose decreased to 9 Gy.
Discussion
In our study, enema performed before cylinder insertion in 38 patients did not influence rectosigmoid Dmax and D2cm3. The enema administration could potentially reduce the rectal dose by reducing the rectal volume.
In literature, results of observations are indecisive. In one study, authors reported that larger rectal volumes were associated with higher rectal dose parameters during VCB [3]. Unfortunately, we did not find a relationship between preparation for the procedure with enema and higher doses in rectosigmoid, which was also confirmed by other authors [4,5]. However, a reduction of rectal doses can be achieved by removal of gas in the rectum during VCB [6]. A significant decrease of the mean rectum volume by 29% and D2cm3 by 11% was observed by Sabater et al. [6]. In our study, we did not measure gas volume in rectosigmoid. The weak point of our study is the lack of CT planning carried out with and without enema in the same patient.
The bladder volume in the range of 27-256 cm3 did not influence Dmax or D2cm3 in our study. Literature data on the analysis of the effect of bladder volume on the dose given during VCB are also inconclusive. In a dosimetric study, Mahantshelly et al. compared the mean bladder volumes of 64.5 cm3, 116.2 cm3, and 172.9 cm3, and a significant increase in small bowel dose in the empty bladder when compared to the full bladder was noted for intracavitary BT in cervical cancer patients. Only non-significantly higher bladder doses in 3 series were observed [7]. Like in our study, bladder filling did not increase the dose in it. The dosimetric effects of bladder filling in patients with VCB with a distended bladder preferentially reduce high-dose to the small bowel around the vaginal cuff without a significant change in dose to the bladder, rectum, or sigmoid, as reported by Hung et al. [8]. On the other hand, in literature, the full bladder produces a significant, 18.7% of bladder D2cm3 increase [9], and an empty bladder in 35 of 45 women reduced bladder doses by 0.5 Gy on average [10]. Hoskin et al. analyzed doses in empty and three full bladder volumes (35, 70, and 100 ml), and showed that the maximum bladder dose was lower with the empty bladder than with any of the full ones [11].
The analysis of Dmax and V100 in OARs is very important. The analysis of V100 in the rectosigmoid revealed large differences in our study. A D2cm3 dose in the rectosigmoid equal to 7.4 Gy was acceptable, but Dmax = 19.7 Gy was not. There is no recommendation concerning Dmax and V100 in OARs in the literature; however, the V100 or Dmax reduction in cases on overdosage is required. Our data based on standard plans, emphasized the benefit of 3D over 2D planning.
This study analyzed doses in the rectosigmoid, not only in the rectum, and it is a possible reason for the slightly higher dose in the organ. We decided not to contour these two organs (rectum and sigmoid) separately due to a short length of source position activation in the cylinder (2.75 cm), to avoid overdosage of OARs. High doses in OARs are observed in patients with thin vaginal walls and coexistent bowel loops adjacent to the apex of vagina. In such cases, it can be considered to prescribe the dose to the applicator surface, not at 0.5 cm depth. The utility of repeated OARs dose-volume histogram calculations in multifractional HDR VCB, using 3-dimensional imaging was assessed by Holloway et al. [12]. Variation of within-patients coefficients of D0.1cm3 and D2cm3 were for the bladder: 14% and 8.1%, for the rectum: 7.9% and 5.9%, and for the sigmoid: 27.6% and 20.3%, respectively. The authors in [12] concluded that the small variation in doses to the bladder and rectum did not support reporting doses to the OARs beyond the initial fraction. In our study, CT was performed only before the first brachytherapy fraction.
Identification of AGs in our material was 83% in the whole length of the vagina, and 24% of AGs were located in the upper 3 cm of the vagina, including the top of the vault. In a meta-analysis of 9 publications, which met the requirements, 67% of patients had at least one air pocket and 59% per insertion [13]. Sapienza et al. in a prospective study reported that 77.2% of AGs were located within the proximal 2 cm of the cylinder, and 81.8% within the proximal 4 cm of the cylinder [13].
The depth of AGs is essential. In our study, 59% of AGs were small (≤ 3 mm), allowing the accepted dose to be given to the vaginal mucosa. In one patient, a 6 mm AG was located in the apex of vagina, adversely influencing dose distribution.
The incidence of AGs depends on the cylinder diameter, the proper fixation, and relaxation during the procedure. AGs reduce the mucosal dose in the treated area. Since the presence of AGs is often found, post-insertion CT can facilitate selection of optimal cylinder size in VCB or allow modification of dose distribution while planning the process. In our material, only some AGs were observed at the apex of the vagina (upper 3 cm).
Conclusions
Vaginal cuff brachytherapy planning with the use of CT is essential. Enema before cylinder insertion does not influence rectosigmoid Dmax and D2cm3. The analysis revealed no bladder volume effect on bladder doses Dmax and D2cm3. A few percent of plans require correction of dose distribution because of hot spots in OARs. For patients with high Dmax and V100 in OARs, it should be considered to prescribe the dose to the applicator surface, or at smaller than 0.5 cm depth.
Most patients have air gaps around the cylinder, which can potentially reduce the mucosal dose. Selection of cylinder should be based on clinical findings. Post-insertion CT can facilitate a selection of optimal cylinder size. In case of major AGs, dose variation optimization of dose distribution is required. AGs less than 3 mm can be disregarded.