Summary
In this original study, we present a new method of dealing with massive thrombus in the coronary artery in patients with ST-segment elevation myocardial infarction, which we call dual protection: simultaneous use of manual thrombectomy with a distal protection device. The presented method is safe and feasible and can be used in any catheterization laboratory.
Introduction
Each ST-segment elevation myocardial infarction (STEMI) is associated with thrombus formation on a ruptured or ulcerated atherosclerotic plaque [1, 2]. While the human body usually copes well with small thrombi, large ones may cause serious problems. Thrombus size is classified according to the Thrombolysis In Myocardial Infarction (TIMI) thrombus grade [3, 4]. A thrombus grade of ≥ 4 is considered massive (high thrombus burden grade ≥ 4) [5]. A massive thrombus (MT) may be formed in large vessels, at a decreased blood flow rate, venous bypasses, or STEMI late presenters [5–7].
The consequences of a massive thrombus may include lack of reperfusion, extensive myocardial infarction (MI) and its complications, increased mortality, subsequent surgical procedures, increased risk of stroke, and embolization of large coronary artery branches [8, 9]. Thrombus management may involve pharmacotherapy, mechanical or manual devices for thrombus removal, protection systems to prevent distal embolization, laser method, specific stenting techniques, or specially designed stents [10–18]. Although there are various treatment options for patients with coronary thrombosis, guidelines for STEMI do not recommend the routine use of manual thrombectomy (MTH), distal protection devices (DPD) or deferred stenting [19, 20].
Treatment of STEMI and MT patients with MTH and DPD (referred to here as double protection (DP)) has not been tested yet.
Aim
This study aims to present DP outcomes in the treatment of patients with STEMI and MT in the infarct-related arteries (IRA).
Material and methods
The analysis concerns a group of STEMI patients, admitted to our hospital within 12 h after the onset of chest pain or with symptoms of persistent ischemia from 12 to 48 h after the onset of symptoms, who were treated with primary percutaneous coronary intervention (PPCI) by the authors. Fourteen patients with MT in the IRA, revealed by coronary angiography, were included in the analysis. Those patients underwent PPCI using DP. The procedures were performed between October 2009 and December 2015.
Thrombus analysis
Thrombi were classified based on coronary angiography, according to the TIMI Thrombus Grade: Grade 0 – no thrombus; Grade 1 – haziness; Grade 2 – a definite thrombus < 1/2 vessel diameter; Grade 3 – a definite thrombus from > 1/2 to ≤ 2 vessel diameters; Grade 4 – a definite thrombus > 2 vessel diameters; Grade 5 – a thrombus causing total occlusion of the vessel [2, 3].
A thrombus grade of ≥ 4 was considered massive. As in the case of a vessel occlusion (TIMI thrombus grade 5), a thrombus may turn out to be small after reperfusion; if so the thrombus was reclassified after passing a guidewire or small balloon through the occluded vessel (Figures 1 A, B).
Figure 1
The procedure using double protection: A – occluded right coronary artery (RCA), TIMI Thrombus Grade 5 (black arrows); B – reassessment of the thrombus after passing a small balloon through the occlusion, TIMI Thrombus Grade 4 (black arrows); C – application of a distal protection system and insertion of a manual thrombectomy device (black arrows); D – stent implantation, thrombi located distally to the balloon (black arrows); E – the thrombus removed from the artery using a thrombectomy device, a filter filled with thrombi (black arrows); F – RCA with TIMI Thrombus Grade 3 and Myocardial Blush Grade (MBG) 3 (black arrows)
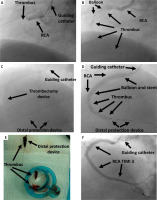
Double protection procedure technique
In STEMI and MT patients, DP involves using DPD as well as MTH as an instrument that reduces the thrombus volume in the vessel to prevent peripheral embolization. DPD ensures safe MTH of the whole procedure especially safe balloon manipulation and stent implantation, protecting against peripheral embolization.
In accordance with applicable European Society of Cardiology guidelines during the study period, all patients were administered with clopidogrel 600 mg or ticagrelor 180 mg before the procedure. Firstly, 50–70 IU/kg intravenous heparin was administered. Then further treatment was conducted under the activated clotting time friquent control. After MT had become clearly visible, patients were administered intravenous abciximab (loading dose of 0.25 mg/kg iv and infusion at 0.125 μg/kg up to 12 h) or eptifibatide (loading dose of 180 μg/kg followed by infusion at 2 μg/kg/min up to a maximum of 18 h).
A DPD – the SpiderFX Embolic Protection Device (Medtronic, Minneapolis, MN, USA), made of a nitinol filter – was applied with a guidewire inserted into the distal part of the IRA (Figures 1 C, 2 A, B). Subsequently, MTH was performed several times using the Diver system (Invatec, Brescia, Italy), Export aspiration catheter (Medtronic, Minneapolis, MN, USA) or Pronto (Vascular Solution Inc., Minneapolis, MN, USA) (Figures 1 C, 2 C). Angioplasty and stent implantation followed MTH (Figures 1 E, 2 D). When thrombi were still visible, the next step was reinserting the thrombectomy device into the vessel and aspirating the remaining residual thrombus (Figures 1 E, 2 E). At the end of the procedure, the DPD was removed (Figures 1 F, G, H, 2 F). After the procedure, coronary artery flow was evaluated according to TIMI and Myocardial Blush Grade (MBG) [21, 22].
Figure 2
Double protection stages: A – passing a guidewire through the occluded artery (black arrows); B – insertion of a distal protection device into the artery (black arrows); C – application of manual thrombectomy (black arrows); D – stent implantation (black arrows); E – re-application of manual thrombectomy (black arrows); F – stented coronary artery after removal of the distal protection device (black arrow)
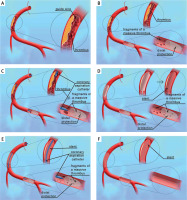
After PPCI, patients were transferred to the coronary care unit. Standard postoperative therapy was used according to the European Society of Cardiology guidelines. In the postoperative period, electrocardiography with ST-segment assessment and echocardiography with the assessment of left ventricular ejection fraction was performed.
All procedures performed in research involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Statistical analysis
Categorical variables were expressed as absolute values and percentages. The normality of the distribution was assessed using the Kolmogorov-Smirnov and Shapiro-Wilk tests. Continuous variables were expressed as mean and standard deviation (SD) for data following normal distribution or a median and interquartile range (IQR) for data with non-normal distribution. All calculations were made using Statistica 10 (TIBCO Software Inc. Palo Alto, CA, USA).
Results
The analyzed group consisted of 14 patients. The clinical characteristics of patients are shown in Table I. Right ventricular MI was found in 2 (14.2%) patients; one was diagnosed with concomitant cardiogenic shock, while another had pulmonary edema. The study group represents 4.8% (13 out of 270) of all invasively treated STEMI patients. The procedural data are shown in Table II.
Table I
Patient characteristics
Table II
Procedural data
Stents were implanted in 13 (92.8%) patients; one patient underwent balloon angioplasty alone due to re-occlusion in a previously implanted stent. Seven (50%) patients had one stent implanted, 4 (28.6%) had two stents, and 2 (14.3 %) had three stents. The mean (SD) stent diameter was 3.6 (0.5) mm. The smallest stent was 2.75 mm whereas the largest one was 4.5 mm. The median (IQR) length of the implanted stents was 25.0 (23.0–63.0) mm. Thrombus characteristics are shown in Table III.
Table III
Thrombus characteristics
All analyzed patients underwent MTH, on average (SD) 3.9 (1.2) times. A DPD was applied in 14 (100%) patients, with a 3 mm size in 8 (57.1%) patients and a 4 mm size in 6 (42.9 %) patients. In 2 (14.3%) patients, angioplasty at the site of the artery occlusion or at the critical stenosis, using a 2.5 mm or 2.0 mm balloon, was needed to apply the DPD. The treatment resulted in complete reperfusion (TIMI flow 3) in 11 (78.6%) patients and TIMI grade 2 flow in 3 (21.4%) patients. MBG was assessed in patients with TIMI flow 3 – grade 3 was found in 5 (35.7%) patients, grade 2 in 4 (28.6%) patients, and grade 1 in 2 (14.3%) patients.
At the end of the procedure, thrombi were observed only in the distal segments of the branches of the infarct artery of 5 patients. These thrombi had a size of Grade 1 to 3 according to the TIMI Thrombus Grade. Three had TIMI flow 2. Control coronary angiography was performed in 5 patients during index hospitalization: in 2 due to the recurrence of ischemic symptoms, in 1 due to cardiac tamponade and in the last 2 was associated with the next stage of revascularization.
The median (IQR) maximal creatine kinase myocardial band was 173.0 (67.6–300.0) ng/ml, and troponin T was 2.3 (0.5–3.6) ng/ml. The median (IQR) maximal ST-segment elevation at admission was 0.45 (0.2–0.7) mV and 0.1 (0.0–0.15) mV after the procedure. Reduction in ST-segment elevation > 50% was obtained in 13 (92.8%) patients. Rapid resolution of ST-segment elevation was not observed in 1 (7.1%) patient. In this patient, follow-up coronary angiography performed on the second day due to cardiac tamponade showed a small thrombus in the medial segment of the RCA.
The mean (SD) left ventricle ejection fraction at discharge was 40.2% (4.1). No myocardial rupture, stroke or death occurred during hospitalization. Recurrent MI was diagnosed in 1 patient, necessitating another percutaneous coronary intervention (PCI). Laboratory tests revealed resistance to clopidogrel. Another patient had a redo PCI of the IRA, did not have an increase in troponin and was not diagnosed with reinfarction. During hospitalization, 2 patients underwent PCI of a non-infarct-related artery. Another patient was diagnosed with cardiac tamponade. The control coronary angiography showed no evidence of bleeding from the coronary artery. Tamponade symptoms caused by an endocardial pacing lead inserted previously were treated with pericardiocentesis. Another patient was diagnosed with gastrointestinal bleeding and required a transfusion of 4 units of blood. All patients survived and were discharged from the hospital. One patient died during the 1-year follow-up.
Discussion
In this study, the analysis concerned MT patients. The minimal thrombus size was 5.5 times longer than the diameter of the artery, and the average length was 12.5 times greater than the vessel diameter. The smallest thrombus in the present study was 20 mm long, and the median length was 39.1 mm. Such large thrombi pose a significant threat to the success rate of PPCI. There is a concern that the coronary artery will not be successfully recanalized and TIMI flow 3 will not be achieved at the end of the treatment. The success rate of PPCI, defined as achieving TIMI 3 flow and dilating the artery while leaving residual stenosis < 20%, was 90–99% in the authors’ previous studies and 80–97% in another study [23–25]. In the present study, DP was applied in the event of MT, i.e., in 4.8% of STEMI patients. According to the authors of this study, an MT is found in STEMI patients mainly when the IRA is the RCA or venous graft, in patients with large arteries > 3.5 mm, and when MI is of long duration. In the present study, 12 out of 14 patients had MI, in which the RCA was the IRA.
MT needs a special management strategy. The contemporary guidelines do not provide clear guidance on how to proceed in such cases. According to the latest guidelines, MTH and DP should not be routinely used in STEMI [19, 20]. This does not mean that they must not be used in particular cases. Studies using thrombectomy suggest that its application may reduce mortality in MI. However, the increased rate of strokes associated with thrombus displacement during aspiration should be taken into account [26, 27]. It largely depends on the procedural technique. DPD did not affect the overall mortality rate among STEMI patients, but it improved secondary endpoints in some studies [14, 28, 29]. DPD has been considered a safe method; however, according to the guidelines, it should not be routinely used either [19, 20]. This method significantly improves the outcomes of coronary artery bypass graft angioplasty [30–32].
Glycoprotein IIb/IIIa inhibitors effectively reduce thrombus volume [33]. They were initially analyzed in patients with non-ST-segment elevation myocardial infarction as a form of adjuvant therapy before invasive treatment and in STEMI patients as one of the treatment strategies – facilitated angioplasty [34, 35]. Finally, after decades of research and experience, their role in the treatment of STEMI patients is currently based on the therapy of thrombotic complications due to angioplasty in the case of large thrombi, and the treatment of the no/slow-reflow phenomenon [20].
The concept of double protection is as follows. In the case of MT, a single treatment method for thrombi in coronary arteries may be insufficient. It is probably reasonable to use several supporting methods. The DPD in MT patients ensures safe MT removal using MTH, without the risk of thrombus displacement, as they would all be caught by the filter of the protection device. Similarly, balloon angioplasty can be safely performed in the thrombus containing lession to carry out thrombus defragmentation and stent implantation without fear that the residual thrombus or small thrombus debris formed during stent implantation will cause a microvascular blockage.
The concerns that DPD embolization passing through MT will result in a peripheral displacement of the thrombus, as well as the fact that sometimes balloon angioplasty must be performed to enable the DP device to pass through a stenosis, seem unfounded. In the case of an MT, the risk of thrombus displacement is low because of the large thrombus volume and frequently peripheral initial location of the thrombus. Therefore, the benefits are likely to outweigh the risks. In our opinion, a DPD should be used at the initial stage of the procedure rather than only after thrombectomy and PCI fail. Kübler presented a similar solution. In the patient presented in this article, however, the DPD was applied after failed thrombectomy [36]. Similar solutions using distal balloon protection systems – PercuSurge Guardewire (Medtronic), TriActive (Kensey Nash Corp.) – and a proximal protection system (Proxis, St. Jude Medical, Minneapolis, MN, USA) were applied in the past. Clinical trials using distal balloon protection systems proved their safety but no effect on infarct size was found [14, 37]. The disadvantages of using the above-mentioned systems include a complete stop of flow in the IRA, the risk of side branch embolization, and the risk of failure to remove all thrombi. In addition, the TriActive system requires 7/8 F guiding catheters. The Proxis system theoretically provides complete protection of the artery from its proximal part to the distal part. However, it does not apply to ostial or proximal lesions because the balloon must be placed in the proximal part of the artery, requires a 7 F guiding catheter, and flow in the artery responsible for MI is completely stopped during its use. The results of a study using the Proxis system in STEMI patients showed no additional benefits of the studied system [38].
Thrombectomy for MT, usually present in large-diameter vessels, may be less successful because of the thrombus volume, the ratio of a vessel diameter to thrombectomy device diameter, and the fact that thrombectomy has limited torquability of its distal part, usually oblique shaped.
Glycoprotein IIb/IIIa inhibitors were found to be beneficial for the treatment of large thrombi, the no/slow-reflow phenomenon and thrombotic complications of PPCI [20]. Interventions of vessels with an already MT are at increased risk of thrombotic complications. Therefore, if an MT is present, glycoprotein IIb/IIIa inhibitors should be used at the initial stage of the procedure. In the present group of patients, glycoprotein IIb/IIIa inhibitors were administered to all patients, except for 1 patient due to existing contraindications. Only 1 patient had symptoms of gastrointestinal bleeding. One patient developed cardiac tamponade, after perforation of the right ventricle by a temporary pacing. No other systemic or severe local bleeding was observed in the remaining patients.
In our opinion, when DPD is applied in MT patients, stent implantation is a safe method. The DP system retains the thrombotic debris formed during implantation, especially in the case of implantation of long stents. If there is a residual thrombus after stent implantation, or the DPD is overloaded, thrombectomy can be performed once again along the entire length of the vessel up to the region where the DPD has been applied. Such therapy is possible in any catheterization laboratory using standard methods (thrombectomy device, DPD and glycoprotein IIb/IIIa inhibitors).
Study limitations. This study has several limitations. It presents the preliminary results of a retrospective study, which were obtained from a single center and involved a small number of patients. Neither thrombi nor flows in coronary arteries and venous grafts were assessed by any independent laboratory. The method needs further research to confirm its success in MT patients. Thirteen out of fourteen procedures were performed by one operator.
Conclusions
DP in MT patients is a safe and feasible procedure. It enables clearing and unblocking of the artery and obtaining TIMI 3 flow in the IRA in a high percentage of patients. However, further observations and studies are needed to assess the efficacy of this treatment method in patients with STEMI and MT in coronary arteries.








