Intensive care units (ICUs) are the focal points for emergence of multidrug resistant organisms [1]. Factors responsible for triggering the emergence of multidrug resistant organisms in critically ill patients include the following: aged > 65 years, prior antimicrobial therapy, or hospitalisation for ≥ 2 days in the last 3 months, in-home wound care, chronic dialysis within the last month and presence of a family member with a resistant microorganism or ongoing immunosuppressive treatment [2]. Several strategies have been shown to be beneficial in optimising antimicrobial therapy in critically ill patients, such as continuous infusion of beta-lactams and vancomycin and using procalcitonin for tapering and discontinuation of the antimicrobial drugs [3]. Novel strategies such as faecal microbiota transplantation and bacteriophage therapy have also been demonstrated as effective in containing and treating multidrug resistant infections in ICUs [4].
ICUs are characterised by an increased workload for physicians, resulting in higher risk of prescribing errors. A study from Brazil revealed that the most frequent omissions in drug description were drug formulation (73.26%) and dose strength (40%) [5]. A prospective 4-year study in Belgian ICUs that included 8,763 participants revealed that 42% of patients receiving antimicrobials had either low or moderate probability of infections [6]. Another study from a developing nation revealed that 86% of antimicrobial prescriptions in the ICU were irrational and that 96.5% were associated with deaths [7]. In the U.S.A., 50% of empirical antimicrobials were administered for prolonged periods lasting beyond 72 hours without any evidence of infection that increased the risk of resistance [8]. Children and neonates are prone to adverse drug reactions considering the developmental changes in their organs, and most of these drugs have not been tested in this subpopulation. In a recent study from the paediatric ICU of a developing country, almost 98% received at least one antimicrobial, of which ceftriaxone was the most administered [9]. A recent systematic review of drug utilisation studies (DUS) in critically ill neonates revealed that they had been carried out only in a few countries such as Brazil (n = 1), the EU (n = 7), India (n = 5), Iran (n = 1) and the USA (n = 6) [10]. There is a dearth of literature regarding the utilisation of drugs in neonatal ICUs in the Middle East. Additionally, there are no studies comparing the utilisation of drugs across critically ill adults, children, and neonates. Hence, we conducted the present study to assess drug utilisation patterns, with a particular emphasis on antimicrobial use in the adult, paediatric and neonatal ICUs of a tertiary care hospital.
METHODS
Study ethics and participants
A prospective observational study was conducted between September 2018 and August 2019 in the adult (AICU), paediatric (PICU) and neonatal (NICU) ICUs of the largest tertiary care hospital in the Kingdom of Bahrain. We obtained approval from the institutional ethics committee (Research Technical and Support Team, Ministry of Health, Kingdom of Bahrain) and written consent from either the study participants or their legal representatives.
Study procedure
Consenting patients were recruited, and information regarding their demographic data, diagnoses, hospital length of stay, outcomes (dead or alive) and the prescribed drug-related details was collected. We excluded intravenous fluids, blood and blood products, vaccines, and total parenteral nutrition from our analyses. The following definitions were adhered to for assessing drug utilisation.
Prescribing indicators were assessed as follows [11]:
Average number of drugs per encounter: This measurement was attained by dividing the total number of prescribed drugs by the number of encounters.
Percentage of encounters with injection prescribed: This value was derived by dividing the total number of encounters with injection by the total number of encounters, which was then multiplied by 100.
Percentage of drugs prescribed from WHO essential drug list: This was attained by dividing the total number of drugs mentioned in the 21st WHO model list of essential medicines by the total number of drugs prescribed, which was then multiplied by 100 [12]. For paediatric and neonatal ICUs, the 7th WHO model list of essential medicines for children was used [13].
Percentage of drugs prescribed from the Bahrain National Formulary (BNF): This measurement was obtained by dividing the total number of drugs mentioned in the BNF by the total number of drugs prescribed [14].
Average duration of prescribed antimicrobial: This measurement was derived by dividing the total number of days in which antimicrobials were prescribed by the total number of antimicrobials prescribed [15].
Daily defined dose (DDD): This value refers to the average maintenance dose per day when used for its main indication in adults as defined by the WHO.
Patient care indicator was assessed as follows [15]:
Average duration of hospital stay of patients receiving antimicrobials.
Lastly, the supplemental indicator was assessed as follows [15]:
The number of antimicrobial drug sensitivity tests reported per hospital admission with curative anti-microbials prescribed.
Moreover, post-hoc, we incorporated estimations of the following variables for antimicrobial use in the paediatric population: antimicrobials prescribed by generic name; antimicrobials prescribed by branded name; antimicrobials prescribed from the 7th WHO Model List of Essential Medicines [13]; antimicro-bials prescribed by intravenous route; days of therapy (DOT); prescribed daily dose (PDD); PDD : DDD ratio; and drug utilisation (DU) 90%. DU 90% was assessed by ranking the antimicrobials in the order of their DDDs and estimating the number of drugs accounting for 90% of use [16].
The WHO list of critically important antimicrobials for human medicine 6th revision was adhered to for classifying the medical importance of antimicrobials [17]. This list classifies the antimicrobial drug classes into important, highly important and critically important. Additionally, for drug classes in the critically important category, two criteria factors and three prioritisation factors were used. Criterion 1 (C1) indicates that the antimicrobial drug class is the only available or one of the extremely few agents for combating serious bacterial infections. Criterion 2 (C2) refers to the antimicrobial class for treating infections by bacteria that may be either transmitted or that acquire resistance from nonhuman sources. Furthermore, prioritisation factor 1 (P1) refers to the antimicrobial drug class with potential to be used for large numbers of people in the community or in certain high-risk populations or those affected by infections with a very limited choice of antimicrobials, whereas prioritisation factor 2 (P2) concerns antimicrobials reported with high frequency of use in healthcare that may favour the development of resistance. Lastly, prioritisation factor 3 (P3) refers to the antimicrobial drug class for treating infections in people for which there exists extensive evidence for transmission of resistant bacteria or resistance genes. Antimicrobials were classified as narrow- and broad-spectrum based on their activity against Gram-positive and Gram-negative bacteria [18–21]. The empirical antimicrobial use was considered prolonged if the antimicrobial drug was continued for more than 3 days despite negative culture and sensitivity results [22].
Statistical analysis
Demographic variables were represented using descriptive statistics. The Kruskal-Wallis H test was used for non-parametric and one-way analysis of variance (ANOVA) for parametric variables. Furthermore, the χ2 test was used for categorical variables. A P value of ≤ 0.05 was considered significant. SPSS version 26 (IBM Corp. Released 2018. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp., USA) was used for statistical analysis.
RESULTS
Demographic characteristics
Four-hundred and ninety-six critically ill patients (196 adults, 100 children and 200 neonates) were recruited for the current study. A summary of various demographic characteristics is represented in Table 1. The diagnoses of the study participants are further listed in Table 2.
TABLE 1
Demographic characteristics of study participants (N = 496)
TABLE 2
Diagnoses of the study participants
Classes of drugs administered in the study population
In total, 2,717, 1,342 and 1,577 drugs were prescribed in critically ill adults, children, and neonates, respectively. Irrespective of age group, most of the drugs were administered parenterally followed by the oral route (Table 3). The median (IQR) for the total number of drugs per encounter was significantly different (P = 0.0001) between adult, paediatric and neonatal ICUs: 11 (8–16), 9 (6–17) and 5 (3–12), respectively. Drugs belonging to alimentary tract and metabolism classes and systemic anti-infective drugs predominated across the different patient age groups (Table 4). The mean (SD) percentages of drugs listed in the national formulary and WHO essential drug list for adult, paediatric and neonatal ICUs respectively were 94.1 (10.1) and 78.4 (12.9); 92.4 (32.4) and 80.4 (15.5); and 80.1 (20.4) and 86.3 (18.7). The percentage was significantly lower in neonates (P = 0.0001) for the national formulary, whereas it was significantly higher in the neonates (P = 0.01) compared to other remaining populations for WHO essential drugs. In all, 32 out of 2,717 (1.2%) adult patients, 9/1342 (0.7%) paediatric patients and 3/1577 (0.2%) neonatal patients in ICUs received fixed-dose combinations of drugs, and the results were statistically significant (P = 0.01). A total of 12,393.6 DDD units of drugs were utilised in AICU, of which drugs belonging to the class of systemic hormones constituted 24.6% (3,045.25 DDD units), whereas alimentary tract and metabolism classes accounted for 23.3% (2,889.29 DDD units). Furthermore, anti-infective drugs for systemic use made up 16.9% (2,092.714 DDD units); blood and blood forming agents, 12.5% (1,544.5 DDD units); and cardiovascular drugs, 11.2% (1,384.1 DDD units). Additionally, respiratory system drugs constituted 5.1% (633.9 DDD units); nervous system drugs, 4.9% (612.755 DDD units), musculoskeletal system drugs, 1.3% (161.56 DDD units); genitourinary system drugs, 0.1% (16 DDD units); anti-neoplastic and immunomodulators, 0.05% (7.5 DDD units); and anti-parasitic drugs, 0.005% (6 DDD units).
TABLE 3
Comparison of routes of drug administration in the study participants
[i] AICU – adult intensive care unit, PICU – paediatric intensive care unit, NICU – neonatal intensive care unit. # – Includes formulations applied on eyes; gels and ointments applied on skin; per rectal formulations acting locally; nebulized solutions; and vaginal pessary. $ – Includes intramuscular, intravenous and transdermal patch formulations. *Statistically significant.
TABLE 4
Comparison of WHO ATC drug classes according to the study population
Antimicrobials administered in the study population
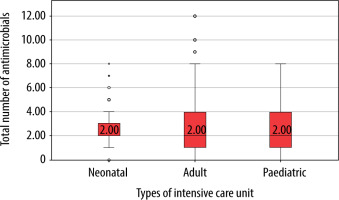
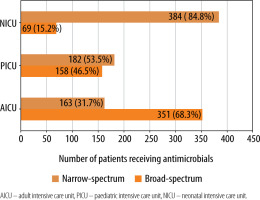
One-hundred and seventy-nine (91.3%) critically ill adults received antimicrobial drugs, whereas 98 (98%) children and 164 (82%) neonates from the respective ICUs received the same. The median (IQR) number of antimicrobial drugs prescribed in adult, paediatric and neonatal ICUs was 2 (1–4), 2 (1–4) and 2 (2–3), respectively (Figure 1) and was significantly (P = 0.02) lower in neonates. The median (IQR) number of intravenous antimicrobials in adult, paediatric and neonatal ICUs was 2 (1–3.25), 2 (1.75–4) and 2 (2–3) and was not significant (P = 0.5). Furthermore, the median number of oral antimicrobials in all the study groups was 0. Moreover, median (IQR) cumulative duration of antimicrobial drugs (days) in the adult, paediatric and neonatal populations was 8 (4–16), 8 (3.5–22) and 10 (6–18), respectively. Critically ill neonates received antimicrobial drugs for a significantly longer duration compared to adults (P = 0.004). A comparison of WHO categories of antimicrobials in the study population is presented in Table 5. A large majority of the antimicrobials were critically important (87.7% in AICU, 83.9% in PICU and 86.5% in NICU). Several of the administered antimicrobials had a combination of WHO prioritisation factors as follows: P1 and P2 (n = 96) and P2 and P3 (n = 17) in AICU; P1 and P2 (n = 34) and P2 and P3 (n = 50) in PICU; and P2 and P3 (n = 269) and P1, P2 and P3 (n = 32) in NICU. Comparison of various antimicrobial drug classes between the AICU, PICU and NICU is depicted in Figure 2. Carbapenems predominated in AICU (96, 18.7%); third generation cephalosporins in PICU (50, 19.3%); and aminoglycosides in NICU (165, 36.4%). Anti-staphylococcal penicillin (6, 1.2%), glycylcyclines (8, 1.6%), polymyxins (17, 3.3%) and tetracyclines (1, 0.2%) were administered only in AICU. Moreover, lincosamides (31, 12%) were administered only in PICU; first generation cephalosporins only in NICU (47, 10.4%); and anti-tuberculous drugs only in AICU (5, 1%) and PICU (4, 1.5%). Figure 3 depicts the comparison of narrow- and broad-spectrum antimicrobials, and significantly (P = 0.001) more broad-spectrum antimicrobials were used in AICU. In AICU, broad-spectrum antimicrobial use amounted to 1,291.1 DDD units, whereas narrow-spectrum antimicrobials totalled 480.4 DDD units. Antibiotics were used definitively in 24 (18.8%), 7 (12.7%) and 18 (12.9%) patients in AICU, PICU and NICU, respectively (P = 0.5). Prolonged empirical antibiotic therapy was observed for 31 (29.8%), 25 (52%) and 51 (41.8%) patients in AICU, PICU and NICU, respectively (P = 0.02). Elevated procalcitonin levels were observed in 24/31 (77.4%), 3/3 (100%), and 4/9 (44.4%) critically ill adult, paediatric, and neonatal populations, respectively. Only one was tested for Clostridium difficile toxin in the stool in AICU and was found to be positive, whereas one patient out of the seven patients sent from PICU had the same finding.
TABLE 5
Comparison of WHO categories of important antimicrobial use in the study population
[i] AICU – adult intensive care unit, PICU – paediatric intensive care unit, NICU – neonatal intensive care unit. *Addition of individual sub-categories may be more than the total as many antimicrobial drugs had multiple criteria. **Comparison of only critically important, highly important and important categories of antimicrobial drugs in each ICU.
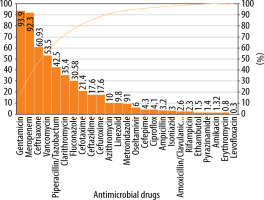
In critically ill children, 180/292 (61.6%) antimicrobial drugs were prescribed in their generic names. Similarly, the median (IQR) number of antimicrobials used in this subpopulation represented in the WHO list of essential drugs was 1 (1–1). Median (IQR) PDD in critically ill children was 0.5 (0.1–1.2), and the PDD : DDD ratio was 0.3 (0.1–0.5). The DOT for antimicrobials was 1,065.63 and that for hospital stay was 1,665 in critically ill children. Antimicrobial utilisation was 64 per 100 patient-days. Gentamicin, meropenem, ceftriaxone, vancomycin, piperacillin/ceftriaxone, clarithromycin, fluconazole, cefotaxime, ceftazidime, cefuroxime, and azithromycin accounted for 90% of use in critically ill children (Figure 4).
Discussion
ICUs are particularly vulnerable to widespread use of antimicrobial drugs due to critical illnesses, complicated most of the time by superseding or similar infections. A multicentric study across 43 ICUs in a developing country revealed inappropriate first-line antibiotic usage in more than one-third of patients with prolonged duration of antimicrobial prophylaxis [23]. A multicentric study from NICU in a developed nation revealed that all penicillin drugs were in the DU 90% segment for all units, that gentamicin was observed in 8 out of 10, that 4 out of 10 used a third-generation cephalosporin (either ceftazidime or cefotaxime), that all except one had vancomycin and that three units had quinolones in the segment [24]. We observed a similar pattern of narrow-spectrum antibiotic use in our critically ill neonates. Patients admitted to ICUs need early and appropriate empirical antimicrobial therapy that can curtail ICU mortality by approximately 70% [25]. On the other hand, inappropriate empirical antimicrobial therapy in terms of either drug selection or duration might increase the risk of antimicrobial resistance [26]. In the present study, critically ill children and neonates received significantly longer duration of empirical antimicrobial therapy. A recent study from NICUs revealed that almost 92.6% of the neonates receiving longer empirical antibiotics had late-onset sepsis, of which 91.2% demonstrated a positive blood culture result, with 95.1% displaying necrotising enterocolitis [27]. However, culture-negative sepsis or localised infections are common in the paediatric population, which leads to heavy consumption of empirical antibiotics [28]. Use of various biomarkers, such as procalcitonin, presepsin, and nCD64, could be valuable in early identification of sepsis [29]. In addition to the conventional C-reactive protein concentrations, a panel of validated novel biomarkers for diagnosis and prognosis of sepsis is essential for critically ill patients [30]. Optimising the use of antimicrobial drugs in critically ill populations is vital considering antibiotic failure due to inappropriate antibiotic use, particularly in patients with sepsis and septic shock, as well as the emergence of antibacterial resistance. Antimicrobial stewardship programmes involving a multidisciplinary team consisting of infectious disease specialists, intensive care physicians, microbiologists and clinical pharmacists might serve to promote more appropriate antibiotic use in this vulnerable population.
The study had a few limitations. First, the illness severity of the study participants could not be assessed, and second, there is a dearth of literature on the appropriate evaluation of DDDs for children and neonates. Therefore, we did not attempt to elucidate this aspect. Furthermore, rationality, appropriateness and the resistance pattern of prescribed antibiotics were not evaluated.