Many civilizational and environmental factors may cause acute or subacute inhalational injury-associated lung diseases [1, 2]. This term covers a diverse group of injuries to the bronchial tree, alveoli, and lung parenchyma; in most cases, such injuries result from inadvertent inhalation of hazardous substances. The extent of lung injury depends on the type, duration and quantity of the substance inhaled, as well as its composition and physicochemical properties (density, solubility). The current knowledge regarding inhalation lung injuries comes mainly from experience in the treatment of fire victims, patients professionally exposed to toxic inhalants, victims of chemical explosions or those excessively exposed to chemical vapours [2, 3]. Inhalation-induced lung injury may result directly from the physical properties of the inhaled agent (temperature, size of a suspended molecule), its chemical structure and direct contact with toxic components of vapours. Hydrogen cyanide, ammonia, formaldehyde, hydrogen sulphide and similar agents represent an obvious risk of lung injury, associated predominantly with the release of proteolytic elastases that stimulate the activation of inflammatory response mediators [2, 4]. Generalisation a localized response leads to the formation of atelectasis foci due to oedema, a decrease in bronchiole diameter, surfactant dysfunction and increased levels of pro-inflammatory factors (interleukin, tumour necrosis factor α). Other agents (e.g. carbon monoxide, cyanide) show generalised effects, including a substance-specific effect on the central nervous system (CNS) and parenchymal organs, resulting from transition through the alveolar-capillary barrier (blood-air barrier) into circulation [1–4]. The accidentality of such mechanisms of respiratory failure confirmed by earlier epidemiological assessments should be revised due to an increasingly high number of reports demonstrating damaging effects of vapours contained in e-cigarettes and other electronic nicotine delivery systems (ENDSs) as well as electronic non-nicotine delivery systems (ENNDS). The use of e-cigarettes is referred to as “vaping”. The Oxford Dictionary defines the verb “to vape” as to inhale and exhale the vapour through the mouth. Informally, vaping is a synonym of using various forms of ENDS, i.e. inhaling nicotine and other vaporised substances. Vaping is becoming increasingly popular, particularly among young people, which is of great concern to epidemiologists and intensive care units (ICUs) due to vaping-associated complications.
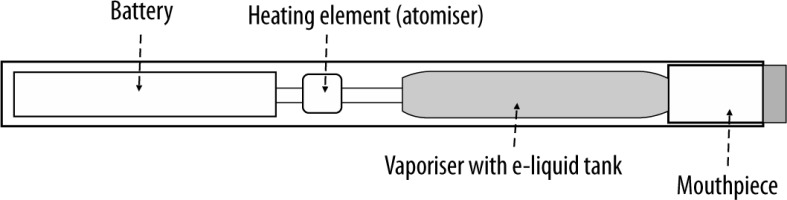
The first attempts to design e-cigarettes were paradoxically made by the Philip Morris tobacco company (the “Premiere” project) in the 60-ties of the 20th century to limit the effects of smoking traditional cigarettes. After many modifications in Asia and Europe, the first e-cigarettes were launched to the US market at the turn of 2006/2007. Since 2014, e-cigarettes have become the most widely used tobacco products among American youths [7–9]. In Poland, the percentage of smokers reported by the Chief Sanitary Inspector is 21% and 1% of the population uses different forms of e-cigarettes [10]. In 2015, JUUL launched a new type of e-cigarette, shaped like the USB flesh drive, therefore, easy to hide from parents. Despite its small size, the nicotine dose in this device is at least 20 times higher than that in traditional cigarettes. The use of such e-cigarettes is called JUULing [7]. Between 2017 and 2018, JUULing increased from 11.7% to 20.8%; in 2018 3.7 million high school students were affected [11, 12]. The observations about vaping in Europe and the United States gradually revealed vaping-associated complications, most commonly lung complications leading to acute respiratory failure (fatal in some cases) [13–15]. The causes of such conditions were comprehensively analysed. It has been demonstrated that e-liquids are mainly composed of seemingly innocent ingredients, e.g. propylene glycol and vegetable glycerine, which are also added to food products and do not exhibit toxic effects when absorbed from the GI tract. However, in e-liquids, they are combined with fragrances and nicotine (0% to 3.6% and sometimes up to 5%) to maintain the moisture and to generate a vapour that simulates tobacco smoke. For this purpose, e-liquid is heated to 150–180°C with a small heater powered by a small battery. In the newer types of e-cigarettes, the temperature can be individually adjusted. Some devices are also equipped with a red diode, lighting up on each aerosol aspiration and imitating cigarette glow [7, 9, 15].
According to the assumptions, the dispersion of vapours is to generate micrometric molecules that are easily inhaled into the bronchial tree. Advocates of vaping stress that e-cigarette aerosol does not contain any of more than 5,000 potentially carcinogenic tar substances present in the smoke of traditional cigarettes. However, the presence of atomised glycol and vegetable glycerine in the respiratory tract is not indifferent. These substances are highly hydroscopic and hyperosmotic, which may cause local inflammatory reactions and generalised effects within the bronchial tree associated with decreased hydration of the gel/sol layer of the muco-ciliary apparatus. Limited capabilities to evacuate bronchial secretions, foreign bodies and microbes, especially in the distal respiratory tract may lead to atelectasis and inflammation. Hyperosmotic properties of both substances and inability to penetrate through the biological membranes, are likely to be one of the factors of expression of pro-inflammatory cytokines and affect the tightness of the alveolar-capillary barrier and production of surfactant, as in the cases of toxic lung injury described above [7, 9, 12, 16, 17]. As a consequence, increased numbers of atelectatic incidents affect the alveoli and small branches of the bronchial tree and are sometimes further complicated by plasma transudation or even penetration of erythrocytes into the alveolar lumen with diffuse alveolar haemorrhage syndrome (DAH) of varying severity [7, 12, 18, 19]. This increases the transpulmonary leakage of unoxidized blood and decreases arterial blood oxygen pressure as well as gas exchange disorders. Additionally, exposed to high temperatures, propylene glycol and vegetable glycerine undergo decomposition, generating potentially harmful carbonyl compounds (acrolein, formaldehyde and acetaldehyde) [8, 12, 20, 21]. The baseline voltage of the current supplying the heating system affects the level of carbonyl compounds found in the aerosol. The carbonyl compounds can trigger typical oxidative stress and stimulate the production and release of inflammatory mediators overlapping the process described above; moreover, they can cause platelet dysfunction. Direct toxicity may also affect the pulmonary endothelial vessels and alveolus structure, leading to rapid gas exchange disorders [9, 11, 20]. Recently, much attention has been paid to the effects of successive heatings of an e-cigarette coil during which nanoparticles are emitted, which can further enhance the negative influence of e-liquid on the airway [9, 12]. In novel generations of e-cigarettes with modified construction and built-in cartomizers, the liquid composition and vaporisation temperature (generation IV mod) can be individually selected and the atomiser parameters adjusted to the smoker’s needs (personalisation). According to American estimates, 700 various e-liquids were available for vaping in 2019; some of them were produced by unauthorised manufacturers. Therefore, the composition of e-liquids significantly varies; some of them do not actually contain nicotine. On the other hand, manufacturers enrich inhalation liquids with adjuvants, the action of which is completely different from the original “safe” supply of nicotine [5, 7, 9, 11, 12, 23]. The agents used, which can injure the respiratory system or affect the CNS include fragrances preferred by users, i.e. alcohols, aldehydes, tetrahydrocannabinol (THC), cannabidiol (CBD), butane-hash oil (called dabs). Moreover, e-cigarettes can contain a variety of opioids (heroin, fentanyl and its derivatives, cocaine, methamphetamine, 3.4-methylendioxyamphetamine (MDA) and 3.4-methylendioxymeamphetamine (MDMA), mephedrone and others). When mixed by unlicensed producers and sold by street dealers, they are likely to contain difficult-to-identify substances whose effects on the human body are unpredictable [7, 14, 16, 17, 24]. An excellent example is e-liquid containing 4-flurobutyrfentanyl which resulted in two first deaths of Polish teenagers [25]. Noteworthy, the majority of the substances described are used during for vaping. Other products may be vaporised. Vaporisation at similar technical parameters to those of e-cigarettes predominantly serves for smoking for marihuana or similar substances heated to 180–220°C. Vaporisation of marijuana is to reduce potential toxicity and increase THC content in the inhaled aerosol [7, 12].
The potential side effects of e-cigarettes, particularly those inducing respiratory and gas exchange pathologies, have resulted in increased surveillance provided by health monitoring and control authorities. According to the Centres for Disease Control and Prevention (CDC), at least 215 vaping-related cases of acute respiratory failure and two deaths were recorded by the end of 2019 in 25 American states. Noteworthy, in November 2019 the country-wide data revealed 2051 acute lung injury cases leading to 39 deaths. At the end of 2019, there were 2172 cases and 42 deaths, respectively (1.9%) [6, 8, 9, 26, 27], which undoubtedly confirms the magnitude of the issue. Although there are no explicit European data, the extent of the problem is likely to be comparable. Almost all patients reporting to the health care centres due to vaping required hospitalisation; more than 60% of them were treated in ICUs, 32% of whom needed intubation and mechanical ventilation. Some patients met the criteria of acute respiratory distress syndrome (ARDS); several patients with severely impaired gas exchange were provided with extracorporeal membrane oxygenation (ECMO) [26–28]. Some cases hospitalized in the ICUs were subacute. Several cases of fibrous pneumonia dynamically developing within a few days were also observed. Moreover, one case of interstitial pneumonia progressing over 6 months was diagnosed, likely to be associated with the heavy metal content in e-liquid [29]. Biochemical tests carried out among 867 patients admitting to using THC-containing e-liquids within 3 months preceding the development of symptoms, demonstrated the presence of potentially harmful substances, e.g. medium-chain triglyceride oil, other lipids and vitamin E acetate. Many recent studies have focused on vitamin E acetate used as a condenser, particularly useful for producing THC-containing liquids. Vitamin E acetate was detected in bronchoalveolar lavage (BAL) samples of all patients declaring the use of THC-containing products. This is the first potentially toxic substance identified in bioptates and BAL from patients with inhalational lung injury, which may be directly associated with lung damage mechanisms. It is likely that more compounds are involved; however, there is no sufficient clinical and analytical evidence to evaluate the interactions [31].
FIGURE 3
Chest computed tomography. A 32-year-old patient without concomitant diseases. History of e-cigarette use
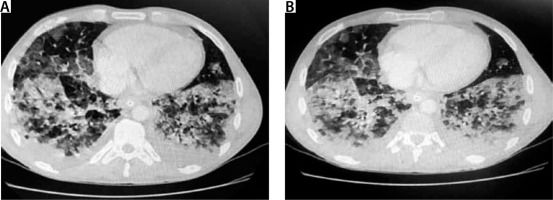
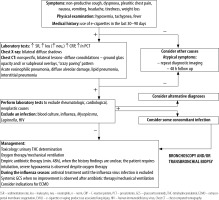
It is not easy to diagnose e-cigarette-associated lung injury, as the initial symptoms are not pathognomonic. Most clinical descriptions stress the development and increasing severity of general symptoms, e.g. dyspnoea, non-productive (dry) cough and chest pain. In addition, gastrointestinal disorders, nausea, vomiting, generalised abdominal pain, and subjective heat sensations are observed. However, symptoms of the upper airway infection are rare; therefore, the diagnosis, often crucial for further treatment strategy, is delayed [11, 12, 17]. Due to vague symptoms, the outpatient broad-spectrum antibiotic therapy is prescribed. The lack of improvement following antibiotic therapy and intensified symptoms of respiratory failure are characteristic of e-cigarette-induced respiratory pathologies. Laboratory analyses demonstrate moderate leucocytosis with a high percentage of neutrophils, increased C-reactive protein (CRP) values at relatively low levels of procalcitonin. Radiologic lung changes are observed in over 90% of all the patients treated and are extremely important [32]. The most common X-ray finding is diverse bilateral shadowing, which is the basis for performing lung computed tomography (CT). Although not characteristic, the CT scan is important for diagnostic considerations. The changes observed are diffuse consolidations, ground glass or crazy paving patterns [8, 9, 12, 33]. In such cases, sputum evaluation and bronchoscopy with BAL should be performed and cellular elements searched for, e.g. lipid-laden macrophages, although eosinophils, neutrophils or lymphocytes predominate in some cases [17, 24, 31]. The presence of lipophages is a noteworthy and quite repeating feature found in the literature cases, though their pathophysiological significance has not been fully elucidated. Some centres have considered their presence a characteristic feature useful for the diagnosis of vaping-associated lung injury; however, recent reports disprove such explicit opinions [9, 12, 35, 36]. The identification of lipophages in sputum, BAL or biopsy specimens, combined with other elements of biochemical and radiological assessment, may become a useful marker of this disease. Furthermore, lipoid pneumonia, often initially diagnosed as interstitial inflammation, can also be confused with ARDS, lung cancer or pulmonary granuloma; therefore, differential diagnosis ought to be extremely meticulous. Lipoid pneumonia can also be exogenous, as in the most bizarre case of fatty fluid absorption by a fire swallower [37]. BAL combined with an open lung biopsy is likely to visualise lung-damaging changes; due to their primary cause, they are acronymically called EVALI or less commonly vaping-related acute lung injury (VpALI) [7, 8, 11, 12]. The consequences of EVALI vary and often depend on individually differentiated reactions to vaping. They may be associated with the development of acute eosinophilic pneumonia, fibrous pneumonia, lipoid (lipid) pneumonia, diffuse alveolar damage (including diffuse alveolar haemorrhage), ARDS, hypersensitivity pneumonitis, also known extrinsic allergic alveolitis, and giant cell interstitial pneumonia [6, 8, 11–13, 17]. Noteworthy, each of these diseases unrelated to vaping is extremely difficult to diagnose. However, it should be remembered that the history of e-cigarettes use can help to determine the cause of a given disease, although the coincidence is not absolute. At present, on the threshold of 2020, however, several elements seem to be likely to facilitate the diagnosis, despite diverse clinical manifestations and non-specific radiological and histopathological images. The majority of patients (> 80%) manifesting lung injury symptoms reported using nicotine, THC or CBD [8, 9, 22]. In the study groups, the concomitance of bacterial and viral infections was excluded, and the symptoms and imaging findings were suggestive of toxic lung injury. As highlighted above, due to mixing of many ingredients with compounds originally intended for inhalation and potential impurities, new compounds are formed. A thorough ex-post analysis has demonstrated that in addition to the above mentioned and expected chemicals, e-liquids contain variably occurring potentially toxic and interacting substances, occurring in variable amounts, such as carbonyls, volatile organic compounds (e.g. benzene and toluene), nanoparticles, trace elements, bacterial endotoxins, and fungal glucans. Moreover, the study findings have revealed that some flavouring substances, e.g. diacetyl, and 2,3 pentanediol, impair the pathway of expression of genes associated with the ciliary cells and cytoskeleton of alveolar epithelial cells, in addition to other components. irrespective of other components [38, 39]. It is also difficult to determine how important the type of e-cigarettes, frequency of exposure (dosage), origin and final composition of inhaled substances are. The available data predominantly pertain to young populations with no history of health issues yet still developing the EVALI symptoms. In each case, patients presented comparable clinical features and disease progression, which implies a similar pathophysiological mechanism of lung injury.
The treatment of EVALI is difficult at each stage due to varied clinical pictures, diagnostic difficulties, and individual respiratory reactions to the damaging factor, which may be extremely rapid [9, 40, 41]. According to the generally accepted literature data, when the features of respiratory failure exacerbate and ICU treatment is required, de-escalating broad-spectrum antibiotic therapy should be initially administered; effective attempts of using doxycycline or azithromycin have also been reported. In most cases, it is not the antibiotic effect of the preparations mentioned above that matters but their known influence on the pulmonary matrix. Noteworthy, steroid therapy is undoubtedly a common element of management options in each case. Unfortunately, clinical descriptions regard various types of preparations, different inclusion times and further therapeutic strategies [4, 8, 9, 12, 13, 20, 42, 45, 46]. In most cases, high doses of i.v. methylprednisolone administered in critical respiratory failure cases are considered the treatment of choice. Milder cases are treated with oral preparations, usually administered for 7–14 days. Passive oxygen therapy based on gasometric results and observations of breathing mechanics can be efficiently supported with high flow oxygen therapy (HFOT) or non-invasive ventilation. In patients requiring invasive forms of mechanical ventilation, lung protective rules of ventilation should be unconditionally followed. The currently known elements of lung pathologies during EVALI pose a significant and specific risk of ventilator-associated lung injury, which results from impaired patency of the bronchial tree in distal branches and surfactant dysfunction. The resultant atelectatic zones significantly disturb the volume distribution and regionalisation of mechanical ventilation with a tendency towards volume injury. An increasing leakage of non-oxidised blood is a logical circulatory consequence of this fact. Based on the current knowledge, pressure-limited ventilation, low tidal volume values (VT 6 mL kg−1), plateau pressure not exceeding 28 cm H2O and PEEP up to 10 cm H2O should be considered mandatory. It is important to maintain driving pressure below 15 cm H2O and provide personalized and tailored ventilation. Imaging diagnostics and physical manoeuvres (including prone positioning) may be required to maintain efficient gas exchange although external CO2 elimination systems might also be needed. ECMO is described as an alternative to ineffective ventilation therapy that should be provided according to the current protocols and therapeutic intuition in cases of increasing severe gas exchange disorders and deterioration of the patient`s condition [7, 8, 12, 19, 20]. Moreover, it is emphasised that haemodynamic monitoring to achieve cardiovascular stability and conservative fluid therapy are needed, especially in cases of EVALI complicated by ARDS.
According to epidemiologists, the use of e-cigarettes is likely to reduce the number of smokers using traditional cigarettes, yet does not decrease the incidence rates of nicotine addiction [7, 10, 17]. The loss of control over the frequency of e-cigarettes use is a dangerous addiction-related factor; nevertheless, the final vaping effects seems to reduce the hazards caused by adverse side effects of traditional smoking [5, 11, 16, 17, 21]. Moreover, the literature contains completely different reports, stressing all the adverse features of vaping, particularly when modified by additional substances. The reported cases of deaths whose aetiology is associated with e-cigarettes, should be a memento. Until the key toxic factors responsible for lung injury are identified, it cannot be explicitly determined which factor exerts the most severe adverse effects on the respiratory system. In clinical practice, it is essential to treat competently the most critically ill patients in the ICU; although the management is largely symptomatic, its earlier application can be effective. Moreover, it is crucial to raise public awareness of the potential risk and harmful effects of any type of vaping [43]. An increasingly high percentage of nicotine-addicted young people should be particularly alarming, more so that the developing CNS is extremely vulnerable to permanent nicotine-related changes. Moreover, vaping used by pregnant women with the intension of limiting the toxicity for a foetus, should be of special concern.
Respiratory failure in the risk group associated with the use of e-cigarettes (especially containing THC) and other illicit substances is a complex and difficult social and therapeutic challenge related to ICU management. At present, adequate dynamics of diagnosis and treatment, optimization of mechanical ventilation and access to alternative ventilatory strategies are the basic standard of management yet does not guarantee fully effective treatment.