Across the globe, inguinal hernia surgery is a frequent surgery. The majority of patients have an effective surgical repair of their inguinal hernias, with some experiencing postoperative pain complications [1]. Additionally, it has a 7–15% negative impact on a patient’s everyday activities and workdays [2]. Effective multimodal analgesic approaches for the treatment of acute postoperative pain may lower the risk of chronic postoperative pain. Simple bedside tests, like quantitative sensory testing, might be used to predict inter-individual variance in the feeling of pain [3]. For interventional pain mana-gement, low-pain responders require straightforward methods, but high-pain responders may require antihyperalgesic medications [4].
Following surgery, the patient feels postoperative pain, which is defined by a variety of unpleasant sensory, emotional, and mental experiences linked to endocrine, metabolic, physiological, and beha-vioural reactions [5]. Even though it varies from patient to patient, the procedure to surgery, and even country to country, the incidence of moderate to severe pain in the postoperative period ranges from 8.4% to 47%. The likelihood of postoperative delirium and cognitive impairment may rise if postoperative pain is not well managed [6].
On the one hand, transversus abdominis plane (TAP) block has already been mentioned as having positive effects on persistent pain following hernioplasty [7, 8]. It is straightforward and treats the pain at its source before centrally mediated alterations can take place, which makes the peripheral approach interesting [9]. Buprenorphine, on the other hand, appears to be particularly supportive because of its distinct pharmacological profile [10]. Furthermore, a dosage of 150–300 μg of buprenorphine is intravenously injected for plexus blockade or peri-pheral nerve block, producing anaesthetic action and a longer duration of analgesia with no discer-nible side effects [11].
Buprenorphine has also been explored for its antihyperalgesic effects [12]; however, research on its peripherally mediated effects has been done in a few studies. Therefore, the study aimed to ascertain how adding buprenorphine to a TAP block with ropivacaine affects both short- and long-term pain characteristics following inguinal hernia surgery.
METHODS
The study and all experimental protocols were approved by the institutional human ethics committee of October 6 University, Egypt (PRC-Me-2102014 dated 02/2021). Additionally, the study was registered in trials.gov (NCT05549492). All experiments were performed in accordance with relevant guidelines and regulations. Male patients with unilateral hernias, ASA I and II certifications from the American Society of Anesthesiologists (ASA), age 18 to 60 years, and a body mass index (BMI) of less than 30 kg m–2 met the inclusion criteria. Any difficult hernia, bilateral hernia, coagulopathy, BMI > 30 kg m–2, patients with a history of persistent pain of any kind, prior surgery, and opiate addiction were all consi-dered exclusion criteria.
The research included 90 individuals who had been treated for elective unilateral open inguinal hernia. Sixty-four patients were randomly assigned to groups B (n = 32) and RB (n = 32) after reviewing the inclusion and exclusion criteria and providing written informed consent. Using randomly gene-rated computer numbers, the patients were divided into 2 equal groups. In sealed envelopes with serial numbers, group allocation was kept secret until immediately before the TAP block administration. Group R received 20 mL of 0.25% ropivacaine for TAP block; group RB received 20 mL of 0.25% ropivacaine containing 300 µg of buprenorphine. An anaesthesiologist who was not a part of the study prepared and coded the study medication.
The day before surgery, the patient’s medical history was taken, and a physical examination was performed. Patients were made aware of the 11-point visual analogue scale (VAS) (0 – no pain, 10 – worst imaginable pain) [13], as well as the 11-point VAS-A (0 – no anxiety, 10 – greatest an-xiety) and 101-point numerical pain scale (0 – no pain, 100 – worst imaginable pain) [14]. Patients were fully aware of the mechanical temporal summation (mTS), preoperative examination, and wound hyperalgesia index postoperative evaluation (WHI) [15]. The magnitude of mTS was calculated for all included patients; mTS was premeditated as the difference between the last and first pain scores. mTS was supposed to be existent if the value of the last stimuli was higher than the first (the value > 0). WHI was calculated at 24 and 48 hours as the sum of distances to the incision from the point of hyperalgesia (cm) divided by the length of the incision (cm). Illustratively, the length of an incision was measured in centimetres at 24 hours in the postoperative ward, and the wound hyperalgesia index was determined using VonFrey filaments (# 6.45 180G) in accordance with standard sterile technique [15]. Stimulation was started outside of the incision from a point where no pain was present and moved inward at 0.5 cm increments towards the wound until the patient complained that it was painful, sore, or sharp. Stimulation was stopped at 0.5 cm from the incision if no changes in sensation were noted. Using a ruler, we marked and measured the point where the incision would be made. Testing was performed along radial lines around the incision separated by 2.5 cm to determine the area of hyperalgesia. This procedure helped to determine how much pain the patient is feeling in the area around the incision, which gives the medical team a better understanding of how to best treat the patient. The WHI is a numerical representation of the pain the patient is feeling, and it helps the medical team determine if the patient needs additional pain management. Patients were asked to rate their degree of anxiety using the VAS-A (visual analogue scale for anxiety). If the patient’s score was 5 or more, they were deemed anxious; if it was less than 5, they were deemed not worried. All patients provided written informed consent.
Intravenous access was set up when the team arrived at the operation theatre. An electrocardiogram and noninvasive blood pressure (NIBP) module were attached and continuously monitored until the surgery was complete. Spinal anaesthesia was administered with a 25G Quincke spinal needle in the lateral position and 3 mL of 0.25% ropivacaine at the L3–L4 interspaces under rigorous aseptic precaution. After 5 minutes, the block level was measured, and surgery was initiated as prescribed in a similar study [16].
After the procedure, the level of the block was noted, and the normal sterile protocol was followed to conduct an ultrasound-guided TAP block on the matching side. To obtain a transverse view of all 3 layers of the lateral abdominal wall, from superficial to deep, i.e. the external oblique, internal oblique, and transversus abdominis muscles, a sheathed high-frequency linear ultrasound probe (6–14 MHz) SonoSite II was placed in mid-axillary line in a transverse plane. An in-plane method was used to insert a 22G 50 mm short bevelled needle from the medial to lateral direction, ensuring that the entire needle was visible during advancement as a bright hyperechoic line aimed at the aponeurosis between the internal oblique and transversus abdominis muscles. To confirm that the needle tip was in the precise location for the hydro-dissection procedure once it had reached the intended fascial plane, 1 mL of 0.9% normal saline was admini-stered.
An anaesthesiologist who was not informed of the patient allocation evaluated the pain ratings postoperatively using the VAS at rest and when the patient was seated at 6, 12, 24, and 48 hours. For up to 48 hours, frequent checks on the heart rate (HR) and NIBP were made. A 50 mg intravenous injection of diclo-fenac sodium was administered as a supplementary analgesic if the VAS was still more than 4.
At 2 and 4 months after surgery, all patients underwent evaluations to determine whether chronic pain had developed at the surgical site, both during rest and when moving. They underwent an interview and completed the Douleur Neuropathique (DN4) questionnaire (the DN4 questionnaire is a 10-item questionnaire with ‘1’ marked as yes and ‘0’ marked as no, allowing the maximal score of 10, with scores more than or equal to 4 corresponding to a higher probability of neuropathic pain) to estimate the onset of neuropathic pain.
The analgesic and antihyperalgesic impact of buprenorphine in comparison to the control group was the study’s main outcome. Evaluations were done of the duration of analgesia, analgesic usage, postoperative pain ratings while sitting and at rest for up to 48 hours, and impact on wound hyperalgesia at 24 and 48 hours. The study’s secondary objectives included the determination of the frequency of side effects and TAP block-related problems. Buprenorphine’s impact on patients who were expected to have high pain scores and the prevalence of chronic postoperative pain were also studied.
To find a clinically significant difference in the length of analgesia between the groups, a power analysis was done. To achieve a power of 90% and a confidence interval of 95%, a sample size of 32 participants in each group was needed. There was a 0.05 alpha error. There were 44 patients in each group to account for dropouts.
SPSS was used to conduct the statistical analysis. For each quantitative variable, the mean and/or median ± SD were determined. The relationship between 2 variables, age and pain ratings, was investigated using the Spearman correlation. The statistical threshold for significance was set at 0.05.
RESULTS
Demographic analysis
The median ages of patients in groups R and RB were 45.23 (8.12) and 44.98 (7.89) years, respectively. The median weight in group R was 68.25 (5.49) kg and in group RB was 69.36 (6.23) kg. In terms of ASA grade distribution, there was no significant difference between the 2 groups. Diabetes mellitus affected 6 patients in group R and 5 patients in group RB, while hypertension affected 4 patients in group R and 5 patients in group RB. There was no significant variation in the distribution of anxiety across the different groups. mTS > 0 was observed in 6 (18.75%) patients in group R and 5 (15.62%) patients in group RB; 26 (81.25%) patients in group R and 27 (84.37%) patients in group RB had shown mTS = 0. There was no significant difference between the various group in terms of the distribution of mTS (P = 0.46), as shown in Table 1.
TABLE 1
Analysis of demographic characteristics
Analysis of operative characteristics
The surgery took an average of 62.32 (13) minutes in group R and 64.56 (12) minutes in group RB. No patient in group RB had a block at the T7 vertebra after surgery. A block was seen in 8 (25.0%) patients in group R and 11 (34.4%) patients in group RB at T8, 16 (50.0%) patients in group R and 13 (40.6%) patients in group RB at T9, and in 8 (25.0%) patients in group R and 8 (25%) patients in group RB at T10, as shown in Table 2.
TABLE 2
comparison between operative characteristics parameters
Analysis of primary outcome
The median duration of analgesia in group R was 386.5 (37.25) min, and in the group RB it was 868 (41.30) min. The difference was statistically significant. Median pain scores at rest were found to be significantly better in group RB than in group R at 6, 12, and 24 hours. At 48 hours, the diffe-rence was not statistically significant between the 2 groups. The median pain score on sitting was found to be significantly better in group RB than in group R at 6, 12, and 24 hours (P < 0.001). At 48 hours the difference was not statistically significant between the 2 groups (P < 0.052). As indicated in Table 3, there was a significant difference in the trend of the pain score (rest) over time in both groups (P < 0.001).
TABLE 3
Comparison of duration of analgesia and pain VAS score between the groups
Comparison of 2 groups in terms of change in pain score (sitting over time)
At 6, 12, and 24 hours, it was discovered that group RB had considerably lower median sitting pain levels than group R. Median pain scores on sitting were found to be significantly better in group RB than group R at 6, 12, and 24 hours (P < 0.001). When the difference between the 2 groups was measured after 48 hours, it was not statistically significant (P = 0.324). Using generalized estimating equations, as shown in Table 4, there was a significant difference between the 2 groups in the trend of the pain score (sitting) over time (P < 0.001).
TABLE 4
Comparison of the 2 groups in terms of change in pain score (sitting over time)
Comparison of the 2 groups in terms of change in WHI over time
WHI changes between the 2 groups were compared using the Generalized Estimating Equations technique. In group B, the median WHI decreased from a maximum of 1.23 at 24 hours to a minimum of 1.00 at 48 hours. This change was statistically significant (P < 0.001), as shown in (Table 5). In group RB, the median WHI decreased from a maximum of 0.09 at 24 hours to a minimum of 0.05 at 48 hours. This change was statistically significant (P < 0.001). Using the Generalized Estimating Equations approach, the WHI trend showed a significant difference over time (P < 0.001) (Table 5).
TABLE 5
Comparison of the effect of wound hyperalgesia between the 2 groups
Incidence of side effects and haemodynamic parameters
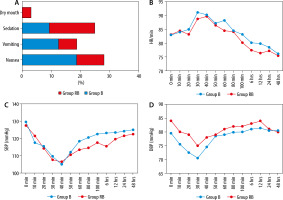
The incidence of side effects was comparable among the groups: 6 (18.75%) patients in group R and 3 (9.37%) in group RB had nausea, 4 (12.50%) patients in group R and 2 (6.25%) patients in group RB had vomiting, and 3 (9.37%) patients in group R and 5 (15.63%) patients in group RB had sedation. Only one patient (3.12%) in group RB had a dry mouth, as shown in (Figure 1A). In the first 8 hours after surgery, every side effect manifested itself. None of the patients had any additional TAP block-related complications. Up to 48 hours, haemo-dynamic metrics were comparable amongst the groups (Figure 1B–D).
Incidence of persistent postoperative pain (PPOP)
At 2 and 4 months, none of the patients in group RB experienced any pain. A score of 4 was assigned to DN4, indicating that the patient suffered from neuropathic pain. However, in group R, the overall incidence of PPOP was 6.25% (2 out of 32 patients).
DISCUSSION
Ultrasound-guided transversus abdominis plane block has been augmented with adjuvant buprenorphine to boost the quality of the analgesia provided by ropivacaine. The study examined the effects of buprenorphine on analgesia, anti-hyperalgesic, and its influence on PPOP following inguinal hernia repair.
According to the current study, ropivacaine and buprenorphine added to a TAP block caused analgesia that lasted longer than it did in the control group. Similar effects of persistent analgesia were shown in the brachial plexus block [17, 18] after the addition of buprenorphine. In addition, compared to the control group, the current study demonstrated a substantial decline in pain levels when sitting and at rest for up to 24 hours. This finding was consistent with a study by Dundar et al. [19], who showed that the adductor canal block with buprenorphine for total nephrostomy tract after percutaneous nephrolithotomy resulted in a comparable decrease in opioid intake. A similar finding was made for infraclavicular brachial plexus block, which showed that perineural infiltration of 100 µg of buprenorphine also demonstrated an extended duration of analgesia and a decrease in pain ratings [20]. Buprenorphine was added to unilateral inguinal hernia repair [21] to prolong the duration of analgesia.
The long-lasting analgesia may be a result of buprenorphine’s strong affinity and binding ability for certain receptors [22]. After attaching to the receptor, it modifies voltage-sensitive calcium channels, alters potassium channels, and decreases the formation of cyclic adenosine monophosphate by modifying the calcium channels [23]. Additio-nally, buprenorphine reduces the excitability of primary afferent neurons by preventing the production of the excitatory neurotransmitter substance “P” and the calcitonin gene-related peptide [24]. It was also proposed that exogenous opioids cause axonal flow at opioid receptors in primary afferent neurons, producing extended analgesia by peripheral action.
A prospective study by Seervi et al. [21] found that adding buprenorphine to 0.25% levobupivacaine decreased analgesic use, postoperative pain score, and duration of analgesia by 1 hour and 5 hours, respectively, following IHR in comparison to perineural dexamethasone and the control group in the TAP block. However, compared to the control group in the current trial, perineural buprenorphine extended the duration of analgesia by 8 hours. This could be explained by the fact that ropivacaine has a lower potency than dexamethasone. Additio-nally, the length of acute pain in the first 24 hours, rather than its severity, predict the likelihood of developing PPOP; therefore, this prolonging of analgesia is advantageous.
While several studies have explored the advantages of immediate postoperative analgesic results, the current study went further to assess the advantages of perineural buprenorphine over the neuroplastic alterations induced by surgery. This finding was consistent with the study by Wheeler et al. [25] in which the peri-incisional mechanical hyperalgesia following open IHR; most people have it for up to 4 weeks before it goes away after 12 to 24 weeks. Furthermore, a similar study was held by Borys et al. [26], who revealed that patients after caesa-rean delivery were provided with both truncal blocks and morphine, which reduced both pain severity and morphine consumption. In the same context, the results of one study showed that patients after open and laparoscopic nephrectomies expe-rienced significant reductions in persistent pain after quadratus lumborum blocks were performed post-operatively [27].
In terms of the difference in WHI between the groups, our data revealed that the WHI’s severity decreased from 24 to 48 hours. Similar studies found that central neuronal sensitization brought on by tissue damage decreases pain [28, 29]. The ongoing release of proteolytic and inflammatory chemicals into the wound tissue sustains strong nociceptive impulses produced during surgery, even after the procedure. After surgery, this impact persists for many hours [30]. However, according to a study by Tverskoy et al. [31], spinal ropivacaine decreased postoperative pain and wound hyperalgesia after hernia surgery and induced nonselective blocking of afferent input. As opposed to ropivacaine alone, perineural buprenorphine in the current study would have successfully inhibited the afferent nociceptive impulses produced after surgery following the influence of the spinal anaesthetic. The incidence and severity of WHI at 48 hours would have decreased much more as a result.
The current study indicated a significantly lower use of analgesics and pain ratings when at rest and while sitting for up to 24 hours. However, the trial by Bollag et al. [32], who examined 75 μg of clonidine added to hyperbaric bupivacaine 12 μg after caesarean section in the control group, found no change in the incidence or severity of WHI after 48 hours. Additionally, there was no discernible difference between the groups in analgesic usage or pain ratings. The mechanism of action of clonidine to lower WH is located in the spinal cord fairly peripherally, and the author indicated that 150 mcg was a negligible amount.
Side symptoms such as nausea, vomiting, and sedation were equally common in either group. As an agonist of the receptor, buprenorphine produces all the effects of opioids, including analgesia, drowsiness, euphoria, and respiratory depression. Only a few trials demonstrated that 300 µg of perineural buprenorphine caused a significant incidence of vomiting [33, 34]. None of the patients in group RB had pain at 2 or 4 months in terms of ongoing postoperative pain. Additionally, the present study’s low prevalence of neuropathic pain contrasts with previous studies’ findings of high incidences of neuropathic pain following abdominal hysterectomy (33.3%) [35], and inguinal herniorrhaphy (38.7%) [36]. However, our study was consistent with the Polat et al. [37] study, which revealed that the effective postoperative analgesia with TAP block following IHR decreased the occurrence of PPOP [37].
A limitation of the present study is the inability to measure buprenorphine’s plasma levels to exclude systemic effects is the first limitation. How-ever, prior studies have demonstrated the perineural effects of buprenorphine as an adjuvant in regional anaesthesia [38]. The second limitation is that due to practical considerations, a small sample size was employed to assess the incidence of persistent pain following IHR. Larger samples would be needed in future research.