During endotracheal intubation (ETI), a difficult airway is a challenging scenario, with the potential to result in hypoxaemia, causing catastrophic consequences for both the patient and the clinicians involved. Preoxygenation before ETI is therefore of paramount importance because it serves to increase the amount of oxygen reserve in the body. This prolongs the buffer time before hypoxaemia occurs, allowing patients to tolerate a longer duration of apnoea. The clinicians in a crisis situation such as this would have a larger margin of safety, allowing them time to think and to act in securing access to the patient’s airway, thus potentially saving the patient’s life [1, 2]. The importance of preoxygenation is such that today it is an essential part of the arsenal available to clinicians performing ETI, and it has been incorporated into many airway management guidelines [3, 4].
The head-elevated position has been recommended to be the optimal position for preoxygenation before ETI [1, 2]. Multiple studies have shown that this position increases the effectiveness of preoxygenation in patients undergoing general anaesthesia by prolonging the safe apnoea period (SAP), defined as the duration of apnoea before hypoxia sets in [5–9]. However, a study by Semler et al. [10] disputed this when they found that in critically ill patients, the ramping position did not improve oxygenation during ETI, as compared to the supine sniffing position.
Currently, although the head-elevated position is widely accepted as a method to improve preoxygenation, there is no systematic review or meta-analysis in the literature investigating this. This systematic review and meta-analysis is the first to explore this topic, and the findings from this study will shed more light on the effectiveness of the head-elevated position in improving preoxygena-tion, thus improving the knowledge base for clinicians worldwide to perform optimal preoxygena-tion for patient safety. The objective of this study was to investigate the effectiveness of preoxygena-tion when patients undergoing endotracheal intubation are placed in the head-elevated position as compared to the supine position, by reviewing randomized, controlled trials.
METHODS
This report was completed by adherence to the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) 2020 statement [11].
Eligibility criteria
Studies that fulfilled the following eligibility criteria were included in the review: (1) Randomised controlled trials (RCT); (2) Preoxygenation in the head-elevated/ramping position versus the supine position, regardless of the measured outcomes; (3) Adult patients (≥ 18 years old) undergoing endotracheal intubation. No language and date restrictions were applied for studies that fulfilled the inclusion criteria. Non-randomised studies, including observational studies, case reports, case series, and conference abstracts, were excluded.
Search strategy
The PubMed, EMBASE, MEDLINE (via EBSCOhost platform), SCOPUS, and Cochrane Controlled Regi-ster of Trials (CENTRAL) electronic databases were systematically searched from their inception until 31 May 2020. We updated the database search again on 29 June 2021 using the same keywords, but we narrowed the searches to June 2020 onwards. We also searched the Clinicaltrials.gov registry and the WHO International Clinical Trials Registry Platform to find ongoing or unpublished trials. Reference lists of narrative reviews on preoxygenation and included studies were also searched for additional relevant articles. The search terms used were “(ramp* OR “head-up” OR position) AND (pre-oxygen* OR preoxygen*)” (Table 1).
TABLE 1
Search strategy
Study selection and data collection
Three reviewers (NLV, JL, CCC) independently screened titles and abstracts of studies obtained from the database search to determine articles for full text review. Any disagreements were resolved by a fourth reviewer (SEHT). Full text articles were then screened by 3 reviewers (NLV, JL, and CCC) to determine their eligibility for inclusion in the review. In the event of disagreements, a fourth reviewer (SEHT) was consulted. The final decision to include studies into the review was made by consensus of all the authors. Data from selected studies were then extracted independently by 2 reviewers (NLV and CCC) using a standardised data extraction form, which was pilot tested beforehand. In the event of disagreements, a third reviewer (SEHT) would make the final decision after discussion with all authors. Extracted data items included year of publication, trial design, type of population, sample size, sample characteristics, interventions given, and outcomes studied. Because there were multiple definitions of the head-elevated position, we predefined this position as the position in which patients’ torsos are elevated from the horizontal level, regardless of the methods used.
The Cochrane Collaboration Risk of Bias Assessment Tool (first version) was used to assess the risk of bias of included studies [12]. Two independent assessors (NLV and JL) determined the level of bias for each domain, with any disagreements resolved by a third author (SEHT) after discussion. To assess for reporting bias, the outcomes specified in trial protocols will be compared with the outcomes reported in the corresponding published trials, and should trial protocols be unavailable, we will then compare the outcomes reported in the methods and results section. The overall level of bias was judged according to the Cochrane Handbook’s recommendations [12]. In the event of unclear information regarding study methodology, we attempted to contact the authors of the respective studies. Certainty of evidence and summary of findings were independently assessed by 2 reviewers (NLV and SEHT) using GRADEpro GDT software to classify evidence as “high”, “mode-rate”, “low”, or “very low”. As recommended by the Cochrane Handbook, 5 criteria were used to judge the certainty of evidence (risk of bias, inconsistency, indirectness, imprecision, publication bias) for each outcome. Considerations for upgrading the certainty of evidence were also made, based on the following criteria: large effect, plausible confounding, and dose-response gradient [12].
Outcome measures
The primary outcome of this review was the duration of the SAP, defined as the time taken for oxygen saturation to fall to a predetermined level after a defined starting point in seconds (s). Secondary outcomes in this review were (1) duration of recovery time for oxygen saturation to rise back to a specified level after the apnoeic period; (2) arterial oxygen tension after preoxygenation; (3) incidence of desaturation or hypoxemia; and (4) incidence of adverse outcomes such as cardiovascular complications (e.g. hypotension/arrhythmias) or cerebrovascular complications (e.g. stroke).
Summary measures and statistical analysis
The effect measures for continuous outcomes and dichotomous outcomes were presented as mean difference (MD) or odds ratio (OR) with 95% confidence interval (CI), respectively. We combined subgroups into a single group when there was more than one method of positioning in the head-elevated group or supine group, and calculated the resulting mean and standard deviation (SD) for the new group [12]. To analyse the pooled data, the inverse variance method was used for continuous data, while for dichotomous outcomes the Mantel-Haenszel (M-H) model was used. We performed a random-effects model for all analyses, considering the heterogeneity in the interventions used in each study. To assess for the heterogeneity of the included studies, we used the I2 statistical test, with < 40%, 40% to 60%, and > 60% categorized as low, moderate, and substantial heterogeneity, respectively. Pre-planned subgroup analyses to identify sources of heterogeneity were carried out by stratifying the studies into population studied (operating room [OR] setting vs. non-OR setting, and obese vs. non-obese). Sensitivity analysis was performed to determine if our results were robust when the included studies were stratified into high risk or low risk of bias. We also planned to generate a funnel plot asymmetry test to assess publication bias. All analyses were carried out using Review Manager version 5.4 (The Cochrane Collaboration, Copenhagen, Denmark). Statistical significance for all outcome measures was set at a 2-sided P-value of < 0.05.
RESULTS
Study selection
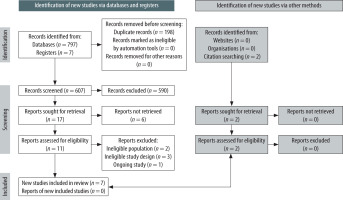
The literature search found a total of 797 records from 5 databases, and 7 records from clinical registries. After screening of titles and abstracts, we attempted to retrieve 17 reports for assessment of eligibility based on the inclusion and exclusion criteria; however, 6 reports were not retrieved because they were from clinical registries (Table 2). A further search from citations of selected studies and reviews yielded 2 reports. From these 13 studies, 6 studies were excluded due to ineligible population [13, 14], ineligible study design [15–17], and status as an ongoing study [18] (Table 3). In total, 7 studies with a total of 508 patients were included into the systematic review (PRISMA flow diagram found in Figure 1).
TABLE 2
Ongoing trials/unpublished studies from registry
TABLE 3
Excluded studies
Study characteristics
The 7 studies included in this review ranged from 2003 to 2020 (Table 4) [5–10, 19]. Six studies were conducted on patients in the surgical operating theatre (OT) [5–9, 19], while one study was done among intensive care unit (ICU) patients [10]. Three studies investigated obese patients [5–7], 2 on non-obese populations [9, 19], and 2 other studies did not specify the body mass index of the recruited patients [8, 10]. In all 7 studies, the intervention groups were placed in a head-up ramping position, ranging from 20° to almost 90°. Almost all studies investigated the SAP as their primary outcome [5–9, 19], with the exception of the study done by Semler et al. [10] in which the primary outcome was the lowest arterial oxygen saturation (SpO2) between induction and 2 minutes after intubation.
TABLE 4
Characteristics of included studies
[i] A-a – alveolar-arterial, ASA – American Society of Anaesthesiologists Physical Status Classification, BMI – body mass index, SpO2 – oxygen saturation on pulse oximeter, FEO2 – fraction of expired oxygen, LMA – laryngeal mask airway, pCO2 – partial pressure of carbon dioxide in arterial blood, pO2 – partial pressure of oxygen in arterial blood, PEEP – positive end expiratory pressure aTime for SpO2 to drop from 100% to 92% after disconnection of endotracheal tube from breathing circuit. bTime from end of thiopentone injection until SpO2 fell to 90%. cTime taken to reach SpO2 92% from time of induction. dTime for SpO2 to reach 97% after initiation of ventilation. e5 patients were excluded from analysis for failing to reach end tidal FiO2 of 85% during preoxygenation. fTime of rocuronium administration until SpO2 falls to 95%. gExcluded from meta-analysis. hTime taken for drop in SpO2 to 93% or apnoeic period of 10 min elapsed (from time of induction). iTime taken for SpO2 to fall to 92% from loss of consciousness.
Risk of bias assessment and certainty of evidence
The risk of bias assessment is summarised in Figure 2. Overall, all included studies had high risk of bias, mainly due to no blinding of personnel to the intervention given. Four studies were judged to be at high risk of bias for allocation concealment because the authors did not specify a method to ensure blinding of investigators to participants’ allocations [7–9, 19]. A summary of findings and the GRADE assessment of the certainty of evidence is presented in Table 5, together with the basis for judgments.
TABLE 5
Summary of findings with GRADE assessment of certainty of evidence
* The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI – confidence interval, MD – mean difference, OR – odds ratio GRADE Working Group grades of evidence:
– High certainty: We are very confident that the true effect lies close to that of the estimate of the effect.
– Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Primary outcome
Six RCTs investigated the effects of the head-elevated position on the duration of SAP when compared to the supine position [5–9, 19]. In total 227 patients were enrolled, comprising both obese and non-obese surgical patients in the OT setting. In the included RCTs, the SAP was defined as the time taken from disconnection of the patient from a breathing circuit (one study) [6] or from induction of anaesthesia (5 studies) [5, 7–9, 19] to when the study participant’s SpO2 dropped to 90% [5], 92% [6, 7, 19], 93% [9], or 95% [8].
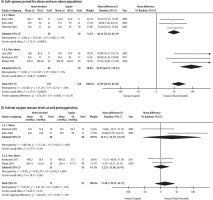
In comparison to the supine position, the head-elevated position significantly increased the duration of the SAP (MD 61.99 s; 95% CI: 42.93–81.05 s; P < 0.00001; I2 = 30%; certainty of evidence = high) (Figure 3). We conducted a subgroup analysis based on obese or non-obese populations to explore for sources of heterogeneity and found that the test of subgroup differences indicated a statistically significant subgroup effect (P = 0.009). The effect of the head-elevated position on the duration of SAP was more marked in the non-obese population (MD 96.93 s; 95% CI: 64.53–129.32 s; P < 0.00001, participants = 119, certainty of evidence = moderate) than the obese population (MD 48.56 s; 95% CI: 31.92–65.19 s; P < 0.00001; participants = 108; certainty of evidence = moderate) (Figure 4). There was no heterogeneity (I2 = 0%) found within pooled analysis in each subgroup. No sensitivity analysis based on risk of bias was performed because all the included studies had a high risk of bias. We did not perform a funnel plot asymmetry test to look for publication bias because there were fewer than 10 studies included in the meta-analysis, and it would lead to an inability to differentiate chance from real asymmetry due to low test power [20].
Secondary outcomes
The recovery time for SpO2 to rise back to 97% after a period of apnoea was only investigated in 2 studies, with a total of 68 patients [6, 7]. Both studies enrolled only obese patients undergoing elective upper gastrointestinal surgeries. There was a non-significant trend favouring the head-elevated position with regards to recovery time (MD –54.90 s; 95% CI: –173.29 to 63.49 s; P = 0.36; I2 = 96%; certainty of evidence = very low) (Figure 3).
Arterial oxygen tension levels (pO2) at end preoxygenation were investigated in 4 studies (n = 170) [5, 7, 9, 19]. There was no difference between the head-elevated and supine positions with regards to end preoxygenation pO2 (MD 13.48 mmHg; 95% CI: –18.35 to 45.31 mmHg; P = 0.41; I2 = 53%; certainty of evidence = low) (Figure 3). Subgroup analysis was performed based on obese (MD 35.31 mmHg; 95% CI: –54.79 to 125.41 mmHg; P = 0.44; I2 = 80%; n = 80) and non-obese (MD 2.23 mmHg; 95% CI: –21.96 to 26.41 mmHg; P = 0.86; I2 = 0%; n = 90) populations, with test for subgroup differences suggesting no statistically significant subgroup effects (P = 0.49) (Figure 4).
Four studies reported the incidence of adverse events when patients were placed in the head- elevated compared to supine positions during preoxygenation (n = 158) [6, 7, 9, 19]. Ramkumar et al. [9] reported hypotension necessitating treatment in the head-elevated group, while Dhakal et al. [19] reported transient premature ventricular complexes among the supine and head-elevated groups. Another 2 studies did not find any adverse events in both groups [6, 7]. Pooled data showed that there was no difference with regards to incidence of adverse events for patients in the head-elevated or supine positions (OR 3.99; 95% CI: 0.50–31.76; P = 0.19; I2 = 0%; certainty of evidence = very low) (Figure 3).
Semler et al. [10] was the only study in this review that studied the effects of head-elevated and supine positions in critically ill patients in the ICU (n = 260). They reported no difference with regards to incidence of hypoxaemia SpO2 < 90% (head- elevated vs. sniffing position, 39.4% vs. 41.7%; P = 0.7) and incidence of hypoxaemia SpO2 < 80% (head-elevated vs. sniffing position, 20.5% vs. 28.3%; P = 0.14). In addition, there was also no difference in the lowest SpO2 between induction and 2 minutes after successful endotracheal intubation (head-elevated vs. sniffing position, median [IQR], 93% [84–99] vs. 92% [72–98]; P = 0.27).
DISCUSSION
To date, this is the first systematic review and meta-analysis done on this important topic, to our knowledge. Through our meta-analysis, we found that in the OT setting, the head-elevated position greatly improved the effectiveness of preoxygenation through prolonging the SAP when compared to the supine position. This effect is greater in the non-obese population than in the obese population. There were no differences between these 2 positions for recovery time to baseline SpO2 after preoxygenation, end pO2 after preoxygenation, or incidence of adverse events.
The prolongation of the SAP by around one minute in the head-elevated position is a very important finding because every second can make a difference during an airway crisis [21]. This effect is mainly caused by the mechanical effect of a raised torso, leading to an increase in functional residual capacity (FRC) [13, 22]. During preoxygenation, the FRC of the lungs stores up additional oxygen, which creates an oxygen reserve that will be spent during apnoea. In the head-elevated position, FRC increases because the weight of the surrounding tissues compressing the thorax is reduced, thereby increasing the compliance of the lung and chest wall. In addition, because of gravity, the pressure exerted by the abdominal contents on the diaphragm reduces, further increasing lung compliance [1, 23]. Another contributing factor for increased FRC in head-elevated posture could be due to reduction in intrathoracic blood volume via blood pooling in the lower extremities, which has been shown to increase total lung capacity and vital capacity in healthy subjects [24]. Together, all these factors lead to an increased FRC and hence increased oxygen reserve in the head-elevated position. Interestingly, we found that the prolongation of SAP in the head-elevated obese population was not as marked as in the non-obese population. This is due to the much lower FRC found in obese patients caused by the greater weight of the chest wall and abdominal fats, leading to reduced total compliance of the respiratory system [25, 26]. However, the prolongation of SAP (additional 48 seconds) afforded by the head-elevated position compared to the supine position can still significantly improve the safety of obese patients during intubation [21].
There was no difference seen in the incidence of hypoxaemia between critically ill patients intubated in both positions. The lowest SpO2 levels peri-intubation also did not differ between both groups. This is probably due to significant differences between patients undergoing elective surgeries and patients in the ICU. Preoxygenation may not have been performed as well in the ICU setting (suboptimal environment, e.g. limited space, poor lighting, suboptimal bed characteristics) compared to the surgical OT setting [27]. Also, the mechanical benefits of increased FRC in the head-elevated group may not have been as prominent among critically ill patients with various lung pathologies. On the other hand, Semler et al. [10] postulated that the head-elevated position may have conferred benefits for oxygenation (as seen in a non-significant trend towards reduced incidence of hypoxaemia and a higher peri-intubation SpO2) but was offset by the longer duration of intubation in the head- elevated position, resulting in no difference between the groups. More research is required in the critically ill population to determine the effects of head-elevated position on preoxygenation.
We did not find any difference between the 2 positions with regards to pO2 at end of preoxygenation. This is consistent with Smith et al. [14], who found that a 45° seated position does not improve tissue oxygenation in patients after a period of preoxygenation. This is probably due to the minimal effect of postural changes on blood oxygen content. In non-hyperbaric conditions, the oxygen content of blood after preoxygenation is already maximal because the haemoglobin is usually 100% saturated in a normal individuals, irrespective of changes in posture. Hence, the benefit of the head-elevated position on preoxygenation is mainly mechanical [28]. No difference was found for recovery times after preoxygenation; however, due to the presence of high heterogeneity between studies, further research is warranted before any conclusions can be made.
The head-elevated position theoretically leads to adverse haemodynamic effects because blood pooling in the lower extremities may lead to reduced cardiac output. This effect may be exaggerated during induction of anaesthesia due to the vasodilatory effects of anaesthetic agents. In our review, we did not find a difference between the head-elevated and supine positions on adverse events such as peri- intubation hypotension. However, this is still an under-investigated area because most human studies are not powered to detect a difference [29]. Of note, a recent RCT showed that the head-up position in swine models may lead to reduced cerebral oxygenation during hypovolaemia, but not during normovolaemia [30]. Until further evidence is available, caution should be taken when placing physiologically vulnerable patients in an excessive head-elevated position for preoxygenation during intubation.
The head-elevated position has been associated with multiple benefits during intubation. This is especially so in the obese population, with evidence showing it can improve laryngeal exposure [31]. Indeed, it has been recommended that intubation of obese patients should be done in the ramped (head-elevated) position [4]. In recent years several studies have reported that the benefits of the head-elevation on improving laryngeal exposure is not limited to the obese population alone, but extends also to non-obese patients [29, 31, 32]. In addition to improving laryngeal exposure, the head-elevated position could also reduce the need for ancillary airway manoeuvres and lead to faster intubation times [33]. The results from our meta-analysis indicating improved preoxygenation effectiveness have added further evidence of benefit for this intubation position.
Our review had several limitations. First, the included studies all had high risk of bias, mainly due to lack of blinding of personnel. This is because of the nature of the intervention, whether head- elevated or supine, which was impossible to blind. Second, in this meta-analysis there was a small number of studies and total patients enrolled, particularly for subgroup analysis. Further large-scale high-quality randomized controlled trials should be carried out to confirm the benefit of the head-elevated position on preoxygenation. Third, we excluded non-randomised studies from this review, which could have led to the risk of publication bias. We accepted this risk to increase the reliability of the findings in our meta-analysis. Fourth, there was unavoidable heterogeneity between included studies due to differences in clinical and methodological factors. We attempted to reduce the effects of hete-rogeneity by running random-effects modelling and performing subgroup analysis to explain the heterogeneity.
CONCLUSIONS
Our meta-analysis showed that the head-elevated positioning for intubation significantly improved the effectiveness of preoxygenation. Prolonging the SAP will provide benefits to patients, especially those undergoing anaesthesia. However, more research is needed to determine if this positioning for intubation has any adverse consequences.
REGISTRATION AND PROTOCOL
This systematic review was registered prospectively in the Prospero International Prospective Register of Systematic Reviews with the registration number CRD42019128962. We updated the protocol in June 2020 to Prospero after a literature search (but published on 29 October 2020) to add another author and to amend our outcome measures. We modified our main outcome to be safe apnoea period, because this was the outcome most cited in RCTs. Other surrogates of preoxygenation effectiveness such as recovery time after apnoeic period and arterial oxygen tension at end preoxygenation were modified to become our secondary outcomes because few studies investigated these outcome measures. In addition, we removed the laryngeal view, success at first intubation attempt, and use of airway adjuncts from our additional outcome measures because no studies compared the ramping with supine positions (sniffing position was used).