Anaemia is common in critically ill patients, and more than one-fourth are transfused with allogenic red blood cell (RBC) transfusions [1, 2]. RBC transfusions can be lifesaving for many patients, but they are also associated with harm such as transfusion-associated circulatory overload (TACO), transfusion-related immune modulation (TRIM), transfusion-related acute lung injury (TRALI), haemolytic reactions, and infections [3]. However, anaemia is also harmful, which makes risk-benefit assessment of RBC-transfusions important and necessary [4]. Many large randomized controlled trials (RCT) with high levels of evidence have demonstrated that a restrictive transfusion strategy (haemoglobin level > 70 g L−1) is as safe as a liberal transfusion strategy (haemoglobin level > 90–100 g L−1) [5–10]. In those RCTs, patients in both groups received RBC transfusions, and many patients may also have been exposed to the risk of anaemia. Consequently, adverse effects related to the low-grade RBC transfusion itself could be difficult to ascertain.
We have recently demonstrated that low-grade RBC transfusions given to septic patients were associated with increased mortality and morbidity in a liberal transfusion setting [11]. Given that RBC transfusions may trigger TRIM, it is possible that harmful effects are more pronounced in septic patients than in other patient groups [11, 12]. To evaluate the harmful effect of RBC transfusions in non-septic critically ill patients who were not exposed to the risks of anaemia, we designed this retrospective propensity score matched study. The aim was to compare mortality and morbidity in critically ill patients without severe sepsis or septic shock, who were given low-grade RBC transfusions at haemoglobin level > 70 g L−1 to those of controls without RBC transfusions in the first 5 days in intensive care. The hypothesis was that RBC transfusions are harmful in non-septic critically ill patients without significant anaemia.
METHODS
Data collection and study population
The study was approved by the Swedish Ethical Review Authority in Lund, Sweden (registration numbers 2014/916 and 2018/866), and the board waived the requirement for written informed consent. The manuscript was prepared according to the STROBE guidelines for observational studies [13].
All patients ≥ 18 years of age, admitted to the 9-bed general intensive care unit (ICU) at Skåne University Hospital, Lund, Sweden between 2007 and March 2018 were eligible for inclusion. For patients with multiple admissions to the ICU during the time of the study, only the first admission was included. To allow significant RBC-transfusions but exclude patients with massive bleeding, patients who received high-grade RBC transfusion (defined as a total of > 670 mL or 2 units per day) during the first 5 days in the ICU were excluded. Day 0 was the day of the admission. All patients with severe sepsis or septic shock according to the Sepsis-2 definition [14] were excluded. RBC transfusions were given at the discretion of the treating physician to maintain a haemoglobin level of 80–100 g L−1 according to local guidelines. To exclude patients exposed to the risks of anaemia, all patients with a pre-transfusion haemoglobin level < 70 g L−1 were excluded.
Mortality data were collected from the Swedish intensive care quality register PASIVA (Otimo Data AB, Kalmar, Sweden). Simplified Acute Physiology Score 3 was registered according to the original publication [15]. Physiological and laboratory data and pre-existing conditions (age, gender, chronic obstructive pulmonary disease [COPD], renal failure), outcome variables (except mortality), and fluid administration data were collected from raw data, i.e. from the electronic master chart system of the hospital (Melior, Cerner, N. Kansas City, MO, USA) or from the patient data management system at the ICU (Intellispace critical care and anaesthesia [ICCA], Philips, Amsterdam, the Netherlands).
Outcome variables
Mortality was assessed at 28, 90, and 180 days after ICU admission, and organ support was assessed by calculating days alive and free (DAF) of organ support for the first 28 days after admission to the ICU. For patients who died in the ICU, we counted the days without the specified organ support before death as previously described [16]. Organ support measures were vasopressors for circulatory failure, invasive mechanical ventilation for respiratory failure, and renal replacement therapy (RRT) for renal failure. Renal failure was also evaluated according to the acute kidney injury network (AKIN) scoring system. The maximal AKIN score the first 10 days after ICU admission was used for analysis. To obtain an overall measure of organ failure we also used the maximum sequential organ failure assessment (SOFA) score during the first 28 days after admission.
Statistical analysis
Patients receiving low-grade RBC transfusion (< 670 mL day−1) during the first five days of ICU admission were propensity score matched with non-transfused patients to adjust for differences in baseline variables associated with outcome. The propensity score was calculated with linear logistic regression using a one-to-many macro for SAS as previously described, with the covariates specified in Table 1 [17]. Physiological and laboratory variables used in the propensity score matching were collected within 90 min of admission to the ICU.
TABLE 1
Patient demographics before and after propensity matching
| Factor | Unmatched groups | Propensity-matched groups | ||||||
|---|---|---|---|---|---|---|---|---|
| Control n = 3949 | RBCa n = 1291 | Standardized difference | P-value | Control n = 674 | RBC n = 674 | Standardized difference | P-value | |
| Pre-existing conditions | ||||||||
| Age, mean (SD) | 58 (19) | 64 (16) | 0.315 | < 0.001 | 62 (17) | 63 (16) | 0.041 | 0.375 |
| Male gender, no (%) | 2342 (59) | 745 (58) | 0.032 | 0.311 | 408 (60) | 392 (58) | 0.048 | 0.375 |
| Blood malignancyb, no (%) | 40 (1.0) | 37 (2.9) | 0.140 | < 0.001 | 13 (1.9) | 12 (1.8) | 0.011 | 0.840 |
| Chronic obstructive pulmonary disease, no (%) | 340 (8.6) | 96 (7.4) | 0.043 | 0.185 | 62 (9.2) | 64 (9.5) | 0.010 | 0.852 |
| Cirrhosis, no (%) | 40 (1.0) | 25 (1.9) | 0.076 | 0.010 | 10 (1.5) | 12 (1.8) | 0.023 | 0.667 |
| Immunosuppressionc, no (%) | 727 (18) | 74 (5.7) | 0.107 | < 0.001 | 31 (4.6) | 33 (4.7) | 0.007 | 0.897 |
| Malignancyd, no (%) | 525 (13) | 296 (23) | 0.250 | < 0.001 | 130 (19) | 131 (19) | 0.007 | 0.890 |
| Nosocomial infectione, no (%) | 138 (3.5) | 48 (3.7) | 0.013 | 0.674 | 26 (3.9) | 26 (3.9) | 0.000 | 1.000 |
| Airway infection, no (%) | 494 (12) | 159 (12) | 0.005 | 0.874 | 93 (14) | 85 (13) | 0.035 | 0.520 |
| Surgeryf, no (%) | 908 (23) | 513(40) | 0.367 | < 0.001 | 231 (34) | 222 (33) | 0.025 | 0.608 |
| Gastro-intestinal-bleeding, no (%) | 71 (1.8) | 25 (1.9) | 0.012 | 0.702 | 13 (1.9) | 13 (1.9) | 0.000 | 1.000 |
| Disseminated intravascular coagulopathy, no (%) | 103 (2.6) | 25 (1.9) | 0.467 | 0.161 | 15 (2.2) | 13 (1.9) | 0.021 | 0.703 |
| Intra-cranial volume effect, no (%) | 123 (3.1) | 38 (2.9) | 0.011 | 0.723 | 16 (2.4) | 17 (2.5) | 0.009 | 0.860 |
| Physiological and laboratory variables at admissiong, mean (SD) | ||||||||
| Heart rate, mean (SD) | 92 (23) | 94 (24) | 0.113 | < 0.001 | 93 (24) | 94 (25) | 0.012 | 0.827 |
| Systolic blood pressure (mmHg) | 126 (30) | 119 (29) | 0.234 | < 0.001 | 120 (29) | 121 (30) | 0.005 | 0.933 |
| Lactate (mmol L−1) | 2.3 (2.5) | 2.4(2.3) | 0.031 | 0.346 | 2.5 (2.6) | 2.6 (2.5) | 0.004 | 0.945 |
| Norepinephrine (μg min−1) | 0.91 (3.2) | 1.8 (5.0) | 0.205 | < 0.001 | 1.6 (4.3) | 2.0 (5.9) | 0.076 | 0.163 |
| Temperature (°C) | 36.6 (1.2) | 36.7 (1.4) | 0.058 | 0.061 | 36.6 (1.3) | 36.6 (1.4) | 0.019 | 0.724 |
| PaO2/FiO2 (kPa) | 33 (18) | 32 (20) | 0.014 | 0.672 | 33 (18) | 32 (21) | 0.049 | 0.368 |
| Leucocytes (× 109 L−1) | 14 (10) | 13 (8.1) | 0.036 | 0.291 | 14 (15) | 14 (8.0) | 0.024 | 0.657 |
| Platelets (× 109 L−1) | 225 (97) | 210 (110) | 0.140 | < 0.001 | 220 (110) | 222 (118) | 0.017 | 0.753 |
| pH | 7.34 (0.12) | 7.35 (0.11) | 0.139 | < 0.001 | 7.35 (0.12) | 7.34 (0.11) | 0.064 | 0.240 |
| Bilirubin (μmol L−1) | 12 (19) | 17 (30) | 0.179 | < 0.001 | 14 (27) | 16 (29) | 0.043 | 0.424 |
| Creatinine (μmol L−1) | 104 (110) | 125 (150) | 0.152 | < 0.001 | 120 (126) | 122 (153) | 0.013 | 0.806 |
| Prothrombin time (PT)/INRn | 1.3 (0.65) | 1.4 (0.72) | 0.141 | < 0.001 | 1.4 (0.76) | 1.4 (0.74) | 0.015 | 0.780 |
| Activated partial thromboplastin time (APTT) (s) | 35 (17) | 39 (19) | 0.227 | < 0.001 | 39 (21) | 39 (21) | 0.009 | 0.871 |
| Hbh | 122 (18) | 105 (15) | 1.037 | < 0.001 | 109 (14) | 109 (14) | 0.008 | 0.882 |
| Circulatory Sequential Organ Failure Assessment (SOFA) | 1.4 (1.1) | 1.8 (1.2) | 0.316 | < 0.001 | 1.7 (1.3) | 1.8 (1.3) | 0.029 | 0.592 |
c Chronic steroid treatment correlative to ≥ 0.3 mg kg−1 prednisolone/day, radiation, or chemotherapy,
e infection that developed after > 48 hours in hospital or secondary to surgical or medical procedure,
The sample size was based on the number of available patients during the study period. Variables were summarized using mean (standard deviation), median (interquartile range, i.e. 25th to 75th percentiles), or numbers (percentage). The propensity score matching was performed by an independent statistician using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) prior to any comparison between the groups. Kaplan-Meier survival analyses were performed, and the results are presented in graphs with corresponding stratified log-rank test. In accordance with previous recommendations, comparisons between the groups after propensity score matching were performed with paired hypothesis testing [18]. Differences between groups over time were compared using the Kruskal-Wallis test, and circulatory SOFA at each of the 5 days were compared using Mann-Whitney U-test. Other analyses were performed with SPSS Statistics version 26 (SPSS Inc., Chicago, Ill., USA). A 2-sided P-value of less than 0.05 was considered to indicate statistical significance.
RESULTS
A consort diagram of all patients is presented in Figure 1. Out of 9491 patients 5240 remained after removing patients < 18 years of age, multiple admissions, high-grade RBC transfusion (> 670 mL day−1), patients with pre-transfusion haemoglobin < 70 g L−1, and patients with severe sepsis or septic shock. After propensity score matching, 674 patients were included in the RBC group and 674 patients in the control group. The annual inclusion rate in both groups was similar (Supplemental File 1). Baseline demographics, comorbidity, clinical, physiologic, and laboratory data in both groups are summarized in Tables 1 and 2. Detailed diagnosis at admission for the propensity score matched groups are presented in Supplemental File 2. After the propensity score matching the standardized differences between groups for included baseline variables were reduced to < 10%. For the baseline variables that were not included in the matching, differences between the groups were eliminated after the matching for all variables except for “Reason for admission, central nervous system” (Table 2).
TABLE 2
Unmatched baseline characteristics
| Factor | Unmatched groups | Propensity-matched groups | ||||
|---|---|---|---|---|---|---|
| Controls, n = 3949 | RBCa, n = 1291 | P-valueb | Controls, n = 674 | RBC, n = 674 | P-valuec | |
| SAPS 3d, median (IQR) | 54 (43–66) | 62 (51–73) | < 0.001 | 60 (48–70) | 60 (50–71) | 0.426. |
| Reasons for admissione, n (%) | ||||||
| Trauma | 272 (6.9) | 122 (9.5) | 0.003 | 40 (5.9) | 50 (7.4) | 0.326 |
| Central nervous system | 1334 (31) | 431 (25) | < 0.001 | 116 (17) | 170 (25) | < 0.001 |
| Haematological | 109 (2.5) | 128 (7.5) | < 0.001 | 20 (3.0) | 24 (3.6) | 0.646 |
| Gastric | 333 (7.7) | 323 (19) | < 0.001 | 67 (10) | 70 (10) | 0.857 |
| Metabolic | 505 (12) | 211 (12) | 0.733 | 63 (9.3) | 66 (10) | 0.853 |
| Respiratory | 1531 (35) | 692 (40) | < 0.001 | 290 (43) | 273 (41) | 0.377 |
| Cardiovascular | 952 (22) | 660 (39) | < 0.001 | 189 (28) | 204 (30) | 0.402 |
| Hepatic | 151 (3.5) | 113 (6.6) | 0.02 | 31 (4.6) | 30 (4.4) | 0.402 |
| Renal | 393 (9.1) | 316 (18) | < 0.001 | 63 (9.3) | 73 (11) | 0.416 |
| Other | 347 (8.0) | 136 (7.9) | 0.853 | 45 (6.7) | 50 (7.4) | 0.670 |
| Arrival route, n (%) | ||||||
| Emergency department | 1885 (44) | 371 (22) | < 0.001 | 192 (28) | 184 (27) | 0.564 |
| General ward | 1106 (26) | 603 (35) | < 0.001 | 205 (30) | 195 (29) | 0.416 |
| Intermediate care | 56 (1.3) | 38 (2.2) | 0.02 | 13 (1.9) | 12 (1.8) | 0.413 |
| Surgery | 564 (13) | 341 (20) | < 0.001 | 123 (18) | 135 (20) | 0.245 |
| Other ICU | 440 (10.2) | 231 (13) | 0.02 | 101 (15) | 102 (15) | 0.844 |
| Other | 237 (6.0) | 90 (7.0) | 0.480 | 40 (5.9) | 41 (6.1) | 0.533 |
e Each diagnostic group as defined in the SAPS 3 original publication [15]. Patients may have more than one reason for admission.
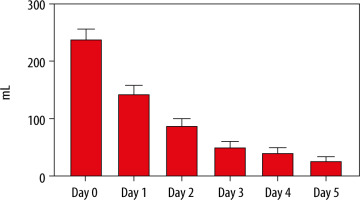
All RBC transfusions were leukoreduced. The median haemoglobin level before transfusion in the RBC group was 98 g L−1 (91–107 g L−1). The median haemoglobin levels before transfusion per year are illustrated in Supplemental File 3. The median haemoglobin level on day 0 was 109 g L−1 (107–112 g L−1) for the RBC group and 109 g L−1 (106–113 g L−1) for the control group (P = 0.96). Daily median haemoglobin levels for the first 5 days for both groups are illustrated in Figure 2. The median volumes of RBC transfusion in the RBC group the first 5 days after admission are shown in Figure 3. The total RBC volume given during the ICU-stay were 0 mL (0–0 mL) for the control group and 595 mL (315–899 mL) for the RBC group.
FIGURE 2
Median haemoglobin level in the 2 groups with interquartile range. There were no differences between the groups over time (Kruskal-Wallis test, P = 0.15). RBC = group with patients who received red blood cell transfusion on any of the first 5 days

FIGURE 3
Mean red blood cell transfusion per day with 95% confidence interval in the RBC group. RBC = group
Outcomes
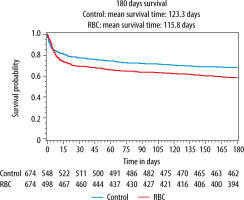
Detailed results are presented in Table 3. Mortality at 28, 90, and 180 days was higher in the RBC group (Table 3 and Figure 4). The absolute risk increase for death at 180 days for patients in the RBC group was 5.9% [95% CI: 3.6–8.3%] (P < 0.001). RRT and AKINmax demonstrated an increased risk for acute renal failure in the RBC group. Low-grade RBC transfusion was also associated with circulatory and respiratory failure as well as higher SOFA-max score.
TABLE 3
Main outcome variables
| Outcome | Propensity-matched groups | Relative risk (95% CI) | Absolute risk increase (95% CI) | P-valuea | |
|---|---|---|---|---|---|
| Control, n = 674 | RBCb, n = 674 | ||||
| 28-day mortality | 151 (22) | 206 (31) | 1.36 (1.14 to 1.63) | 8.1% (3.5 to 13%) | 0.001 |
| 90-day mortality | 188 (28) | 244 (36) | 1.37 (1.17 to 1.60) | 9.8% (4.9 to 15%) | 0.001 |
| 180-day mortality | 212 (31) | 281 (42) | 1.33 (1.15 to 1.53) | 10% (5.1 to 15%) | < 0.001 |
| Renal replacement therapy | 16 (2.4) | 56 (8.3) | 3.50 (2.03 to 6.04) | 5.9% (3.6 to 8.3%) | < 0.001 |
| AKINc | 0 (0–0) | 0 (0–1) | < 0.001 | ||
| DAF of vasopressors | 28 (24–28) | 26 (9–28) | < 0.001 | ||
| DAF of mechanical ventilation | 27 (22–28) | 25 (6–27) | < 0.001 | ||
| SOFA maxd | 6 (4–9) | 8 (5–10) | < 0.001 | ||
FIGURE 4
Kaplan–Meier curves of 180-day survival in the control group (blue line) and the RBC group (red line) (P < 0.001, stratified log-rank test). RBC = group with patients who received red blood cell transfusion any of the first 5 days
To investigate if different trajectories in illness in the days after the matching may explain the results, 2 different sensitivity analyses were performed. Firstly, a shorter exposure time was applied, where patients receiving low-grade RBC transfusion (< 670 mL day−1) during the first day after admission were propensity score matched at a ratio of 1 : 1 to controls without RBC transfusion during the first day after admission. The matching was good with a standardized difference < 10% for all variables. After propensity score matching, 477 patients were included in the RBC group and 477 patients in the control group. The differences between the groups were essentially unchanged compared to the main analysis. For details, please see Supplemental File 4. Secondly, circulatory SOFA day 1 to 5 was compared between the groups. The median score was 1 (1–3) for both groups all of the days and there were no differences between the groups on any of the days.
Fluids
There was no difference either in the median daily administration of colloids, crystalloids, or total fluid balance between the groups. The daily median total fluid administration and urinary output was larger in the RBC group compared to the controls (Table 4). The total fluid balance during the length of stay was +2300 mL (360–3900 mL) for the control group and +2700 mL (210–4100 mL) for the RBC-group, P = 0.094.
TABLE 4
Fluid therapy, first 5 days
| Fluids per daya | Propensity score matched groups | P-valuec | |||
|---|---|---|---|---|---|
| Control, n = 674 | RBCb, n = 674 | ||||
| Median | IQR | Median | IQR | ||
| Colloidsd (mL) | 180 | 0 to 610 | 290 | 140 to 680 | 0.078 |
| Crystalloidse (mL) | 1200 | 260 to 2600 | 1200 | 890 to 3600 | 0.133 |
| Fluids in, totalf (mL) | 2900 | 1600 to 4600 | 3100 | 2500 to 5200 | < 0.001 |
| Urine output (mL) | 1900 | 880 to 2600 | 2100 | 910 to 2900 | 0.040 |
| Total fluid balanceg (mL) | 340 | 50 to 2100 | 710 | −10 to 1800 | 0.111 |
| RBC-transfusion (mL) | 0 | 0 to 0 | 290 | 110 to 400 | < 0.001 |
DISCUSSION
In this propensity score matched study, low-grade leukoreduced RBC transfusions given to non-septic critically ill patients without significant anaemia were associated with increased mortality, increased kidney, circulatory, and respiratory failure, as well as higher SOFA-max score.
We collected data from 2007, prior to many high-quality RCTs recommending a transfusion threshold of 70 g L−1. Altogether, most patients in the RBC group were transfused at a “safe” haemoglobin level without being exposed to the risks of anaemia, indicated by a median pre-transfusion haemoglobin level of 98 g L−1 (91–107 g L−1). Although the haemoglobin level before transfusion demonstrated some variation over time, as illustrated in Supplemental File 3, it was still within a safe non-anaemic limit throughout the study period. These data can therefore be used to evaluate the effect of RBC transfusion itself on critically ill non-anaemic, non-septic patients.
Previous large RCTs have demonstrated the safety of a restrictive transfusion strategy [5–10]. However, it should be noted that most patients in both the low- and high-threshold arms in those RCTs received RBC transfusions. Furthermore, any positive effects of fewer RBC transfusions in the low-threshold group may be offset by a longer exposure of anaemia as compared to the high-threshold group. In the present study the haemoglobin level did not differ between the groups and patients in the control group were neither given any RBC transfusions during the first 5 days after admission nor significantly exposed to anaemia. The current study is more likely to be biased given its retrospective nature, but the methodological differences described above imply that the current study adds further knowledge to the risk-benefit assessment of RBC-transfusions in the critically ill non-septic patients.
The propensity score-matching was performed to minimize the differences in baseline variables between the groups and to create the RBC and control groups as similar as possible at ICU admission. Differences between the groups in variables not included in the matching, such as SAPS 3, disappeared after the matching, with the exception of “Reason for admission, central nervous system” (Table 2). This further underlines the validity of the propensity score matching.
In the present study the exposure time was set to 5 days. The decision to transfuse could reflect differences in the trajectory of the disease some days after admission, which was not matched for. This can be exemplified in the present study with the risk that patients who deteriorate some days after admission may be more likely to receive RBC transfusions than patients who improve. To assess this potential confounder, 2 different sensitivity analyses were performed. Firstly, a shorter exposure time of 1 day was applied prior to a propensity score matching. Secondly, differences in circulatory SOFA score between the groups on day 1 to 5 was investigated. The results after the first sensitivity analysis with shorter exposure time were largely the same as in the main analyses (Supplemental File 4), and there were no differences between the groups in circulatory SOFA the first 5 days. Moreover, there were no differences in the total fluid balance between the groups the first 5 days (Table 4). This all suggests that neither differences in the trajectory of the disease between the groups nor shortening the exposure time explain our findings.
Given that propensity score matching corrected for differences between the groups and that median haemoglobin level in the first 5 days of ICU admission did not differ between groups (Figure 2), the results in the present study imply that any adverse effects of the RBC transfusion are responsible for the worse outcomes in the RBC group. This has previously been suggested in several reports, studies, and guidelines [1, 3, 4, 11, 19–22]. In a retrospective registry study, similarly to the present one, Leal-Noval et al. [4] included moderately anaemic non-bleeding critically ill patients and matched patients who received an RBC transfusion with non-transfused patients. Hospital mortality, number of ICU re-admissions, number of nosocomial infections, and incidence of acute renal failure were lower in the non-transfused group. In contrast to the present study, the pre-transfusion haemoglobin level was not reported and patients with nadir haemoglobin level > 95 g L−1 were excluded from that study. Because the patients in the present study were transfused at a higher haemoglobin level and thus not exposed to the risk of anaemia, and because the results showed an even stronger correlation between RBC-transfusion and worse outcome, this further strengthens the evidence that RBC transfusions should not be given to non-anaemic critically ill patients.
The reasons RBC transfusions are harmful for non-anaemic non-septic critically ill patients remain elusive, but as mentioned above, known adverse effects of RBC transfusion include TACO, TRALI, and TRIM. Given that the total fluid balance between the groups did not differ (Table 4), TACO is a less likely explanation. Even if TRALI is the leading cause of direct transfusion-related death, it is a rare event reported to occur in 1 case in 6000 to 600,000 transfusions [23]. Also, TRALI is most common after plasma transfusion, which makes this an unlikely cause of worse outcome after RBC transfusion in the present study. RBC transfusions contain many different immunomodulatory mediators that interact with and alter immune cell function in-vivo. The effect of these interactions may be both proinflammatory and immunosuppressive but are seldom obvious at the time of transfusion [24]. Nevertheless, these immunomodulatory properties of RBC transfusions may be detrimental over time for critically ill septic and non-septic patients and may be responsible for the results in the present study.
Finally, it is worth noting that our study has some limitations. These include its retrospective nature, which by default makes conclusions regarding causality uncertain. While baseline characteristics affecting outcomes were cautiously adjusted for and differences in disease trajectory were evaluated in a sensitivity analysis, we cannot rule out residual confounding. For example, cardiac output data and plasma lactate at the time of the transfusion could have differed between the groups. Moreover, the single-centre design may limit the external validity of our results. Strengths include the fact that no patients in either group were exposed to the risk of anaemia because patients with pre-transfusion haemoglobin level < 70 g L−1 were excluded. This suggests that outcomes were less biased by any negative effect of anaemia. Furthermore, all physiological and laboratory variables and many pre-existing conditions were registered prospectively in electronic charts and collected as raw data directly from these electronic charts.





