Introduction
Psoriasis is a chronic, relapsing, inflammatory skin disease, affecting 0.91% to 8.5% of adults and estimated 125 million people globally [1, 2]. According to current knowledge, psoriasis is considered as systemic disease associated with a variety of comorbidities, including cardiovascular disease (CVD), metabolic syndrome, diabetes mellitus (DM) or non-alcoholic fatty liver disease (NAFLD) [3, 4]. The main basis of comorbidity in psoriasis is not only chronic systemic inflammation, but also angiogenesis, genetic and immune background, and also oxidative stress, numerous bioactive molecules or proatherogenic lipid profile [3, 4]. Despite a lot of effort that has been put into searching for markers of psoriasis, the knowledge and clinical usefulness remain inconclusive. Therefore it is purposeful to search for pathogenetic pathways leading to development of psoriasis and other inflammatory diseases, as well as newer biomarkers, helpful in predicting and inhibiting the early cardiometabolic disorders (CMDs) and better therapeutic outcomes.
The Krüppel-like factors (KLFs) are a family of transcription factors regulating diverse biological processes that include proliferation, differentiation, survival, growth, development and responses to external stress [5, 6]. KLFs are deoxyribonucleic acid-binding transcriptional factors with the competency to bind CACCC or GT box deoxyribonucleic acid (DNA) elements. Furthermore, they can act as activators or repressors of transcription [6, 7]. The regions of KLFs that do not participate in DNA binding are highly different and take part in protein-protein interactions. Three conserved zinc-type Cys2-His2 zinc fibers at the carboxy-terminal end of the KLFs bind to sites rich in GC in the promoter of target genes. The transactivation and the transrepression domains are located at the amino-terminus of KLF proteins. The name “Krüppel-like factor” was derived from the homologous Drosophila melanogaster protein Krüppel, which means “cripple” and is related to development [7]. The original member of the mammalian KLF family was discovered in red blood cell lineage and plays the main role in the β-globin expression and erythrocyte development. KLF subtypes have different biological functions and impact on numerous diseases. To date, 18 KLFs have been identified, which are divided into three main groups depending on structural features and transcription roles. They have gained a lot of attention due to their participation in metabolic pathways and maintenance of homeostasis of many organs [7]. KLFs are critical regulators of physiological systems including cardiovascular, digestive, respiratory, hematopoietic and immune ones. Their altered expression or activation has been linked with metabolic abnormalities, and has been shown to be involved in the development of diseases such as obesity, CVD, DM, NAFLD or cancer including their key role in the control of vascular inflammation [7, 8]. An increased KLF2 expression has anti-inflammatory and anti-atherosclerotic effects but also inhibits angiogenesis, which in turn is crucial in psoriasis [1, 7]. KLF4 was first described in 1996 as gut-enriched Krüppel-like factor (GKLF) [9]. It is expressed in immune cells, takes part in cell cycle arrest and suppresses apoptosis, however may change its function to pro-apoptotic [6]. Furthermore, it is mainly produced in epithelial cells, not only of the gastrointestinal tract, but also in the epidermis, taking part in differentiation and maintaining the skin barrier [10]. Segre et al. observed deaths of the KLF4 knockout mice due to the rapid loss of body fluids highlighting the role of KLF4 in regulating cell proliferation of the skin epithelium [11]. Madonna et al. noted a reduced expression in psoriatic keratinocytes comparing to healthy ones [12]. Kim et al. demonstrated a significantly higher elevated KLF4 expression in psoriatic lesional skin vs. non-lesional or healthy skin [10]. They suggested the outcomes as a physiological mechanism to maintain the homoeostasis but also due to the immunological interplay with interferon γ (IFN-γ) and tumour necrosis factor α (TNF-α), finally one of the key cytokine in psoriasis pathogenesis [10]. In 2012, Tsoi et al. demonstrated a list of notable genes from the newly identified psoriasis loci and one of them was KLF4 (9p.31.2) [13]. Recently, in 2020, an upregulation of the KLF4 gene in psoriatic keratinocytes has been proved [14]. Therefore the studies cited encourage to deepen research on KLF4 in psoriasis. Other link in that interplay is an important role of the factor in vascular wall biology. KLF4 exerts a function similar to KLF2 protective function in endothelial cells, but even more intensely through different mechanisms such as inhibition of endothelial inflammasome activation or cholesterol flux [8]. It stimulates anticoagulant activity and fatty acid oxidation, followed by the activation of peroxisome proliferator activated receptors (PPARs), which are also highly relevant in psoriasis [7, 8]. Enhanced insulin resistance, vascular inflammation and diet-induced obesity have been demonstrated in animal models with myeloid-specific deletion of KLF4 [15]. Furthermore, a decreased KLF4 expression in monocytes of patients with coronary artery disease (CAD) was demonstrated [16]. Increased serum KLF4 levels were significantly related with the presence and severity of CAD [17]. It can be suspected that KLFs may be another link between CVD and psoriasis. Further, KLF4 induces the IL-17 expression which also takes part in immunological pathways of psoriasis [18].
There are promising reports on the use of various therapeutic methods (including mTOR pathway inhibitors, statins, retinoid agonists and PPARs) which, by modulating the expression of KLFs, may reduce the risk of CVD [19]. The described relationships of Krüppel-like factors with systemic diseases indicate that they might be potential risk indicators for the development of CMDs in psoriasis. However, there is a lack of data explaining the precise role of KLFs in that dermatosis.
The numerous biological functions of KLF4 mentioned above underline our desirability of deepening research into the predictive and diagnostic value of selected KLFs in psoriatics.
Aim
This is the first study that explored serum KLF4 levels in patients with active plaque-type psoriasis and its relations with the disease intensity, inflammation or metabolic indicators and also determined the impact of systemic therapy. Additionally, we attempted to assess the relationship of KLF4 with parameters predicting CMDs occurrence in psoriatics, improving or monitoring the effectiveness of treatment or identifying novel therapeutic targets.
Material and methods
The case control study was conducted on thirty-four patients with active psoriasis vulgaris and fifteen sex-, age- and body mass index (BMI)-matched healthy volunteers. The patient group consisted of 22 males and 12 females, at the mean age of 47.6 ±18.12 years. Persons with other types of psoriasis, pregnancy, inflammatory conditions, autoimmune, oncological or cardiometabolic diseases were excluded from the study. None of the participants was under any dietary restriction. Psoriatics were not on any systemic antipsoriatic treatment 3 months prior to the enrollment. The same investigator assessed the psoriasis area and the severity index (PASI) in all subjects. The study group was divided according to severity of psoriasis into two subgroups: mild-to-moderate (PASI I) with PASI < 10–20 including 23 psoriatics and severe form (PASI II) with PASI ≥ 20 achieved by 11 subjects. All participants were also divided into subgroups depending on BMI, group 0 meant the healthy controls, BMI 1 was related to normal weight (BMI 18.5–24.9 kg/m2) and consisted of 8 psoriatics, group 2 – overweight (BMI 25–29.9 kg/m2) present in 18 subjects, BMI 3 (BMI > 30 kg/m2) comprised 8 obese patients. C-reactive protein (CRP), complete blood count (CBC), serum glucose, total cholesterol (Chol), high-density lipoproteins (HDL), low-density lipoproteins (LDL) and triglycerides (TG), transaminases were tested prior to and after treatment. The type of the drug was chosen by the dermatologist, with regard to disease severity, indications and the clinical or therapeutic history of the patients along with agreement with them. The patients started with two systemic medications given orally: 14 subjects methotrexate (MTX) 15 mg/week using folic acid supplementation (15 mg/week, 24 h after MTX intake) and 20 psoriatics received acitretin (ACY) at a dose of 0.5 mg/kg/day. The treatment lasted 12 weeks. All participants signed informed consent forms before the initiation. The study had the approval of the local Bioethical Committee (Protocol number R-I-002/429/2017) and was consistent with the principles of the Helsinki Declaration.
Serum collection
Fasting venous blood samples were collected under complete aseptic precaution from healthy subjects and patients before and after 12 weeks of treatment using vacutainer tubes with clot activator. Samples were centrifuged at 1000× g for 20 min and preserved at –80°C until assay for KLF4, which was measured using enzyme-immunoassay (ELISA) kit supplied by Quantikine® R&D Systems, Minneapolis, USA with sensitivity of 0.054 ng/ml. Optical density was read with a microtitre plate photometer at 450 nm. The concentrations were assessed by interpolation from calibration curves prepared with standard samples provided by the manufacturer. All the tests were taken by one person in standardized laboratory conditions.
Statistical analysis
Statistical analysis was performed using GraphPad 8 Prism and StatMate 2 software (GraphPad Software; La Jolla, California, USA). The normally distributed data were presented as mean and standard deviation, while the non-Gaussian data as median (full-range). Shapiro-Wilk W test was used to determine distribution. Continuous variables were analyzed using ANOVA, independent sample with Student t-test or non-parametric Mann-Whitney test. Categorical variables were analyzed using χ2 test (chi-square test). The correlations were analyzed using Spearman’s rank correlation analysis. Multiple regression analysis was performed using a stepwise model with a forward elimination procedure to determine the combined influence of variables on particular parameters of the measured system. Multiple regression analysis was performed based on earlier outcomes of Spearman’s rank correlation analysis with additional checking of the residuals normality. After statistical analysis all the results were subjected to checking of the statistical power of performed analysis. Presented data had at least 75% power to detect a difference between means of a high scientifically significance level (α) of 0.05 (two-tailed). A two-tailed p < 0.05 was considered as statistically significant, and non-significant (NS) when p > 0.05.
Results
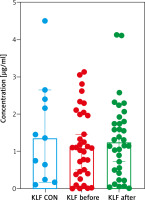
Clinical, demographic and laboratory characteristics of patients and controls are listed in Table 1 and Supplementary materials. A total of 34 patients with active psoriasis vulgaris (12 women/22 men) and 15 age-, sex- and BMI-matched healthy subjects were recruited to the study. The median of basal PASI score was 16.3 (8.4–33.6) points and 10.2 (4.8–22.8) after treatment. In our study group, 23 psoriatics had a mild-to-moderate form of the disease and 11 were diagnosed with severe psoriasis. The family history of psoriasis among patients was positive in 14 (40%) cases. The median value of the serum KLF4 level in psoriatic patients was 1.09 ng/ml and the difference was not statistically significant in comparison to the controls – 1.36 ng/ml (p > 0.05) (Figure 1). However, after dividing the study group regarding the psoriasis intensity, KLF4 was significantly lower in patients with a severe form (PASI II) comparing to the controls (p < 0.05) (Figure 2). KLF4 did not correlate with the severity of psoriasis evaluated by total PASI (p > 0.05) (Table 2). According to demographic data, no significant relations between KLF4 and sex were found (p > 0.05), however in relation with age an upward trend was noted (Table 2). There were no significant relations between the protein and cardiometabolic indices such as liver enzymes activity or glucose or lipids levels (p > 0.05) (Table 2). However, a significant positive statistical correlation between KLF4 and BMI was noted (p = 0.038) (Table 2). Of the investigated basic inflammatory parameters, no statistical significance with KLF4 was noticed (Table 2). However, strong independent relations between particular pro-atherothrombotic parameters were found after multivariate linear regression analysis (Table 3).
Table 1
Basal characteristics of the patients group before and after total treatment and after both drugs separately
| Characteristics | Before treatment | After treatment | After acitretin | After methotrexate |
|---|---|---|---|---|
| WBC [× 103/ml] | 7.59 ±2.14^ | 7.01 ±1.82^ | 7.59 ±2.08^ | 6.32 ±1.01**^ |
| RBC [× 103/ml] | 4.65 ±0.53^ | 4.67 ±0.54^ | 4.68 ±0.48^ | 4.72 ±0.58^ |
| PLT [× 103/ml] | 228.8 ±66.26^ | 232.4 ±57.92^ | 233.9 ±57.25^ | 232.5 ±62.53^ |
| Chol [mg/dl] | 169.6 ±28.3^ | 179.9 ±32.14^ | 180.5 ±27.61^ | 180.2 ±39.52^ |
| HDL [mg/dl] | 46.71 ±11.93^ | 47.66 ±19.3^ | 50.2 ±21.11^ | 42 ±14.62^ |
| LDL [mg/dl] | 103.6 ±23.27^ | 106.2 ±26.5^ | 104.6 ±26.82^ | 110.5 ±26.62^ |
| TG [mg/dl] | 125.4 ±47.69^ | 148.5 ±71.76^ (p = 0.08)~ | 134.7 ±63.85^ | 173.6 ±78.3*^ |
| Glucose [mg/dl] | 84 (66–163) | 88 (69–250) | 88.5 (72–250) | 88 (69–110) |
| ALT [U/l] | 17.5 (8–72) | 18 (7–113) | 17 (9–113) | 18.5 (7–87) |
| AST [U/l] | 19 (12–59) | 18 (11–114) | 20.5 (14–114) | 17 (11–77) |
| CRP [mg/l] | 3.05 (1–58.68) | 1.9 (1–15.2)* | 1.89 (1–15.2)* | 2.05 (1–5.1)* |
| PASI | 16.3 (8.4–33.6) | 10.2 (4.8–22.8)** | 10.15 (4.8–21.9)* | 10.25 (6.3–15.3) |
*/** The existence of a statistically significant difference between values after and before treatment with p-value < 0.05/< 0.001, respectively.
^ ± 1 SD, non-Gaussian data as median (full-range) in brackets. PASI – psoriasis area and severity index, RBC – red blood cells, PLT – platelets, WBC – white blood cells, TGs – triglycerides, HDL – high-density lipoproteins, LDL – low-density lipoproteins, CRP – C-reactive protein, ALT – alanine transaminase, AST – asparagine transaminase.
Table 2
Correlations between baseline parameters and KLF4 in sera of the study group before and after total treatment and both drugs separately
| Parameter | R (p-value) | |||
|---|---|---|---|---|
| Before treatment | After treatment | After acitretin | After methotrexate | |
| PASI | –0.203 (NS) | –0.105 (NS) | –0.454 (0.048)* | 0.533 (0.042)* |
| BMI | 0.382 (0.038)* | N/A | N/A | N/A |
| CRP | –0.101 (NS) | –0.545 (0.0011)* | –0.517 (0.022)* | –0.566 (0.037)* |
| WBC | 0.049 (NS) | –0.248 (NS) | –0.143 (NS) | –0.622 (0.019)* |
| RBC | 0.221 (NS) | –0.248 (NS) | –0.05 (NS) | 0.201 (NS) |
| PLT | –0.243 (NS) | –0.482 (0.0074)* | –0.299 (NS) | –0.572 (0.036)* |
| Chol | 0.133 (NS) | 0.027 (NS) | –0.137 (NS) | 0.134 (NS) |
| HDL | –0.197 (NS) | 0.001 (NS) | –0.012 (NS) | –0.154 (NS) |
| LDL | 0.138 (NS) | –0.087 (NS) | –0.178 (NS) | –0.06 (NS) |
| TG | 0.145 (NS) | 0.312 (0.072)~ | 0.173 (NS) | 0.522 (0.022)* |
| Glucose | 0.121( NS) | 0.246 (NS) | 0.092 (NS) | 0.266 (NS) |
| ALT | –0.027 (NS) | –0.051 (NS) | –0.23 (NS) | 0.057 (NS) |
| AST | 0.024 (NS) | –0.270 (0.098)~ | –0.062 (NS) | –0.531 (0.022)* |
| Age | 0.291 (0.084)~ | – | – | – |
| Sex | 0.072 (NS) | – | – | – |
Table 3
Parameters that independently predict the levels of KLF4 – main effects (A) and two-way interactions (B)
| A | |||
|---|---|---|---|
| Parameter | |t| Value | P-value | P-value summary |
| TG | 3.651 | 0.0218 | * |
| HDL | 3.81 | 0.0189 | * |
| WBC | 3.732 | 0.0203 | * |
| PLT | 5.032 | 0.0073 | ** |
| Glucose | 2.906 | 0.0439 | * |
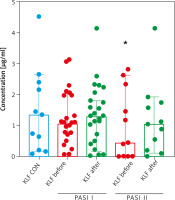
Figure 2
Comparison between KLF4 concentrations depending on the psoriasis area and severity index (PASI) before and after treatment and controls
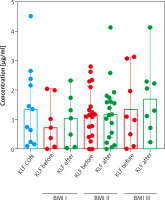
After dividing the study group according to BMI, a downward trend of KLF4 in normal-weight psoriatics with almost half the value as compared to controls and the increasing trend with body weight, especially visible in the group of obese ones comparing to the controls was noted, but they did not reach statistical significance (Figure 3, Supplementary materials). Regarding selected relations inside the BMI subgroups of patients we found that KLF4 negatively correlated with psoriasis severity in normal-weight ones, but not significantly (p = 0.078) (Supplementary materials). A negative trend between the protein and platelet count in obese psoriatics was observed (p = 0.082) (Supplementary materials).
Figure 3
Comparison between KLF4 concentrations depending on BMI before and after treatment and controls
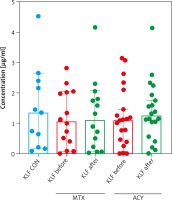
After 12 weeks of systemic therapy skin lesions have significantly improved (p < 0.001) (Table 1). After dividing the patients taking the particular drug, a better, significant improvement after acitretin (p < 0.05) than methotrexate was noted (p > 0.05) (Table 1). Noteworthy is that a significant increase in the TG level occurred after MTX and not acitretin (Table 1). Beside clinical effects of the therapy also limitation of inflammation expressed in a significant decrease in serum inflammatory parameters levels as CRP and white blood celss (WBC) (only after MTX) was noted (Table 1). The median value of KLF4 level in patients after total treatment did not statistically change in comparison to the initial one and of the controls (both p > 0.05) (Figure 1). Similarly, considering the impact of the particular drug, no significant changes in the KLF4 level were noted, beside a subtle upward tendency after both acitretin and methotrexate (both p > 0.05) (Figure 4). Regarding subgroups of patients, no significant changes in the KLF4 level were found after therapy in particular BMI subgroups, but in psoriatics with a severe form the initial statistically lower KLF4 level lost its significance, raised over twice, but still was insignificant comparing with the controls (Figure 3, Supplementary materials).
Figure 4
Comparison between KLF4 levels before and after treatment regarding patients undergoing therapy with a particular drug comparing to the controls
Assessing the relations after total treatment with laboratory parameters, strong negative correlations between the KLF4 level and CRP (p = 0.0011) or platelet count (p = 0.0074) appeared (Table 3). An upward trend between the protein and triglycerides level (p = 0.072) and downward trend with aspartate aminotransferase (AST) activity (p = 0.098) was noted (Table 3). After therapy with acitretin a negative significant correlation of the KLF4 level with PASI (p = 0.048) and CRP (p = 0.022) appeared (Table 3). After treatment with methotrexate we observed some positive relations between KLF4 and PASI (p = 0.042) or triglycerides (p = 0.022) (Table 3). Furthermore, negative significant correlations with CRP (p = 0.037), WBC (p = 0.019), platelets (PLT) (p = 0.036) and AST activity (p = 0.022) appeared (Table 3).
Discussion
Psoriasis is a systemic disease closely related with CMDs mainly through metabolically-driven inflammation. Recent papers published by our team and others showed that psoriatics still require discoveries of new markers of the disease as well as underlying conditions related to the psoriatic progression [1, 20]. KLFs have been an area of intense research lately and thus they constitute a huge excitement for their potential role in medicine, in multiple diseases, not only CVD, but also psoriasis. It has been widely confirmed that KLF4 is a crucial anti-thrombotic and anti-inflammatory factor [7, 8, 21].
To the best of our knowledge, this is the first study evaluating serum KLF4 concentrations in patients with psoriasis and what raises its value, the relationship with systemic treatment. Obviously, conducting the discussion on our outcomes is challenging and hence we will also rely on the results of diseases other than psoriasis, but still related. We demonstrated that the serum KLF4 level did not significantly differ in psoriatics compared with the healthy volunteers. No relation between the factor and disease severity was noted. As cited previously, Kim et al. demonstrated overexpression of KLF4 in the lesional skin [10]. On the contrary, Madonna et al. reported significantly reduced KLF4 levels in psoriatic keratinocytes [12]. The methods used differed, therefore those data cannot be equally discussed. Similarly, with our ones, for the first time serum of psoriatics was evaluated. Although, in the absence of similar data, we cannot draw clear conclusions, the formerly published studies together with own presented point to an uncertain role of KLF4 in psoriasis and perhaps its comorbidities. Presumably, in serum of psoriatics some other confounding mechanisms take part followed by some methodological nuances, small sample sized study group or other ethnical, environmental or lifestyle influences. We found that the KLF4 level positively correlated with BMI. Additionally, we noted a downward trend in normal-weight patients and an upward one in obese psoriatics. These results did not reach statistical significance, presumably in case of larger BMI-subgroups it could be achieved. These data reflect a close relation between KLF4 and adiposity, which is crucial in psoriasis, or could be a compensatory mechanism in response to the metabolically-driven inflammation or perhaps be due to some kind of resistance to KLF4 induced by obesity. Wang et al. conducted a study on omental adipocytes culture, rat models but also on humans [22]. In obese persons the mRNA expression levels of KLF4 were significantly lower than in the normal-weight subjects and they significantly and negatively correlated with BMI, TG, and TNF-α [22]. These results are contradictory to our own ones, however they evaluated the mRNA expression of KLF4 not serum level and the study groups were totally different. Definitely further larger studies are needed to elucidate these divergent mechanisms.
We did not find any relations between the serum KLF4 level and metabolic or inflammatory indicators. However, we found that in multivariate linear regression analysis particular cardiometabolic indices were strongly and independently related with the serum KLF4 level. This undermines that KLF4 might be certainly linked with pro-atherothrombotic risk assessment in psoriasis. As for demographic and clinical data, our results did not show any relation between KLF4 and gender of the subjects studied, however an upward trend with age was observed.
What is meaningful, serum KLF4 concentration was significantly higher in most diseased patients. These outcomes suggest a potential role of KLF4 in the interplay between the intensity of the inflammatory process and the increased risk of CMDs in psoriatics with the greatest skin involvement. Notably, KLF4 might be a relevant biomarker of the proatherogenic risk in severe psoriasis, which needs to be further confirmed. Assuming, in milder psoriasis some kind of physiological homeostasis seems to be maintained. Within the intensifying inflammation in more severe cases it is interrupted and possibly a cascade of pro-inflammatory cytokines or oxidative stress is being overexpressed and up-regulates the serum KLF4 level. It has been confirmed that overexpression of that factor in endothelial cells enhances anti-thrombotic genes, while decreased KLF4 results in the increase in TNF-α-induced expression of vascular cell adhesion molecule-1 (VCAM-1) or E-selectin and thus promotes vascular inflammation [6, 21]. Therefore, gathering this knowledge and our outcomes it would be worth looking for new therapeutic methods that could raise KLF4 in psoriasis. There are reports on drugs that regulate KLF expression. It has been shown that rapamycin, simvastatin, lovastatin, mevastatin and resveratrol induce the KLF2 and KLF4 expression in endothelial cells via different pathways leading to vasoprotection and contributing to prevention of CVD [19, 23, 24]. The positive effect of such a commonly administered drug as simvastatin has been widely documented to date in psoriatics. Recently, Trong et al. has demonstrated that adding oral simvastatin to antipsoriatic therapy might be beneficial in controlling hyperlipidemia and decreasing the severity of the disease [25]. Going further, an improvement in psoriatic lesions has been observed in diabetic patients treated orally with troglitazone and pioglitazone which are PPAR-γ agonists [26, 27]. Lately, Wang et al. has demonstrated that pioglitazone down-regulated the gene transcription of apelin by stimulating transcription of KLF4 in the apelin promoter what leads to prevent diabetic macroangiopathy in type 2 DM rat models [28]. As mentioned above, KLFs, particularly KLF4, stimulate PPAR-γ receptors, thus this interplay additionally highlights that their agonists could be helpful in reducing CMDs in psoriasis. Valuable research was conducted by Garvey et al. [29]. They demonstrated a remarkable remodeling of cyclosporine-A (CSA) treated arteries as well as up-regulation of KLF4 immunostaining in vascular smooth muscle cells and endothelial cells [29]. These outcomes encourage to extend the research with use of CSA, as one of well-established antipsoriatic drugs, on the KLF level and inhibition of vascular inflammation in psoriatics.
To date, there have been no investigations on the effect of systemic antipsoriatic treatment on the serum KLF4 level. The only one convergent study we have found was of Kim et al. [10]. They noted after two months of treatment, a significant increase in the intensity of KLF4 mRNA expression, however the relative one was insignificant comparing to the controls [10]. We are the first ones who demonstrated no significant influence of therapy with methotrexate or acitretin, in total and separately, on serum KLF4 concentration in psoriatics compared with the healthy subjects. However, primary significantly elevated KLF4 in severe patients decreased over twice after systemic therapy, losing its significance vs. controls. We might assume that those classic drugs are insufficient for up-regulating the KLF4 level and its great anti-inflammatory and anti-atherogenic properties, what would be desirable. Furthermore, it does not contradict the CVD predictive value of the factor or antipsoriatic therapy, however there must be other underlying and modulating mechanisms to be elucidated.
We proved that the KLF4 level cannot be a useful marker of effectiveness of psoriasis treatment. Interestingly, after total therapy some correlations have appeared. The serum KLF4 level was negatively related with CRP, PLT and WBC in the MTX-subgroup. These data are puzzling, it could be assumed that KLF4 is a less sensitive inflammatory marker in psoriasis than CRP or perhaps KLF4 is insufficiently up-regulated. Further intriguing results were a significant TG increase after methotrexate and not acitretin, considered more lipids disturbing, which appeared also to be negatively related with KLF4. Hypothesizing, the factor could be a predictor of hypertriglyceridemia in psoriatics undergoing MTX or this drug should be considered as a modulator of pathways dependent on KLF4 in psoriasis, however this requires further research.
The limitations of our study are a small number of patients and controls enrolled, although the groups were relatively uniform in terms of biochemical and clinical data. Additional sub-dividing of an already small study group reduces the reliability of analyses obtained. On the other hand, trends observed in BMI-subgroups point to a potential significance in our future larger research. Thus, our outcomes should be considered as preliminary data. However, we believe that presented results will stimulate further effort to fully elucidate the role of KLFs and particularly KLF4 in psoriasis, the interplay with its comorbidities and treatment together with the discovery of novel therapeutic targets.
Conclusions
Considering the limitations of the study, the lack of data from the literature to refer to, the results obtained cannot be conclusive and final. However, they are undoubtedly valuable and point the way forward to clarify the precise role of KLF4 in psoriasis and its interactions with parameters involved in CMD occurrence. For the first time this study evaluated serum KLF4 levels in psoriatics which were not significantly different comparing to the healthy controls. However, this does not exclude KLF4 as an indicator of comorbidities in those patients. Knowing anti-inflammatory and anti-thrombotic properties of KLF4 and the results obtained, it can be presumed that the factor may serve as an independent marker of the risk of vascular disorders development especially in patients with severe psoriasis or obesity, which are characterized by increased KLF4 concentration. Considering a positive correlation with BMI, KLF4 might be also related with adiposity in psoriasis and thus with systemic metabolically-driven inflammation. Although no significant changes occurred after therapy, the interplay between KLF4 and particularly methotrexate needs in-depth research. The results obtained, promising data on other commonly used drugs up-regulating KLF4 followed by further more intense understanding of KLF impact on various processes should lead to attempts to develop new targeted therapies, also in psoriasis.