Purpose
Low-dose-rate (LDR) brachytherapy (BT) monotherapy has been considered for more than 20 years as the standard of care for patients with low-risk (LR) prostate adenocarcinoma (PCA) [1], with good long-term quality of life [2]. Gradually, guidelines have expanded the use of BT monotherapy to selected intermediate-risk (IR) PCA (Table 1).
Table 1
Existing guidelines for brachytherapy monotherapy for the treatment of patients with intermediate- risk prostate adenocarcinoma
| AFU [13] | EAU/ESTRO/SIOG [1] | ABS [6, 7] | ASCO/CCO [8] | NCCN [9] | |||
|---|---|---|---|---|---|---|---|
| T1-T2 and only 1 unfavorable criterion | Only 1 unfavorable criterion | Only 1 unfavorable criterion | |||||
| Stage | T1-T2 | cT1b-T2a N0 M0 | T2b-T2c | NS | T2b-T2c | ||
| PSA (ng/ml) | 10-15 | ≤ 10 | 10-20 | < 10 AND 7 (ISUP NS) | 10-20 AND 6 | 10-20 | |
| GS | 7 (3 + 4) | 6 AND ≤ 50% | 7 (3 + 4) AND ≤ 33% | 7 (3 + 4) | 7 (3 + 4) | ||
| %PBC | NS | AND PBC < 30% without perineural invasion | AND PBC < 50% | ||||
[i] AFU – Association Française d’Urologie, EAU/ESTRO/SIOG – European Association of Urology/European Society for Radiotherapy and Oncology/International Society of Geriatric Oncology, ABS – American Brachytherapy Society, ASCO/CCO – American Society of Clinical Oncology/Cancer Care Ontario, NCCN – National Comprehensive Cancer Network, GS – Gleason score, %PBC – percentage of positive biopsy cores, NS – not specified
In France, a consensus has been raised in 2004 by French urology societies (AFU), radiotherapy (SFRO), medical physics (SFPM), and pathology (SFP) to propose BT alone to selected patients with only one unfavorable factor, including a PSA 10-15 ng/ml or a Gleason score (GS) 7 (3 + 4), or cT2c [3]. In 2010, the AFU recommendations added a role of magnetic resonance imaging (MRI) to exclude extracapsular extension and evaluate prostate volume, which is the only recommendation to date to mention it and emphasize the importance of the percentage of positive biopsy cores (PBC) for better patients’ selection [4].
In Europe, the 2013 EAU/ESTRO/SIOG guidelines specified that BT was an option for patients with cT1-T2a, GS ≤ 7 (3 + 4), prostate specific antigen (PSA) ≤ 10 ng/ml, and PBC ≤ 50%. It was mentioned that in ‘well-selected patients’ with IR-PCA, long-term data of low-dose-rate brachytherapy were ‘promising’ [5]. The amended version from 2018 defined a more restrictive consensus on the following eligibility criteria for BT monotherapy: stage cT1b-T2a N0, M0; GS 6 (International Society of Urological Pathology [ISUP] grade 1) with PBC ≤ 50%, or GS 7 (3 + 4) (ISUP grade 2) with ≤ 33% of invaded biopsies, an initial PSA level of ≤ 10 ng/ml [1].
The American Brachytherapy Society (ABS) recommended in its 2012 update that “certain IR patients with otherwise low-risk features, such as low-volume disease, predominant pattern 3, and only one adverse feature can be effectively treated with BT alone” [6]. They relied on a survey of BT practitioners, who would all perform BT alone for IR patients, GS 7 (3 + 4) or PSA 10-20 ng/ml, with cT1c and PBC ≤ 30% in the absence of perineural invasion [7]. The American Society of Clinical Oncology/Cancer Care Ontario Joint Guideline, updated in 2017, suggests that LDR-BT alone may be offered as monotherapy for low-intermediate risk prostate cancer (GS 7, PSA < 10 ng/ml or GS 6, PSA 10-20 ng/ml) [8]. The National Comprehensive Cancer Network (NCCN) guidelines recommends that BT alone is an option for patients with very low, low, or favorable IR (FIR) prostate cancer, depending on life expectancy [9].
The aim of this study was to investigate potential predictive factors using EAU guidelines selection criteria in selected IR localized PCA patients treated with iodine-125 (125I) BT alone in our center.
Material and methods
We acquired an IRB approved (R201-004-035) database of patients treated with 125I BT alone for PCA from January 21, 2003 to May 14, 2013 treated at the Centre Léon Bérard Radiotherapy Department.
Patients were classified as IR-PCA according to NCCN classification with at least one unfavorable criterion, such as PSA ≥ 10 ng/ml, ISUP group > 1 (GS > 6), or ≥ T2b disease, with the exclusion of ≥ T3 and ISUP group > 3 and PSA > 20 ng/ml [9]. The study focused on patients with PSA < 15 ng/ml and ISUP group < 2.
Individual patients’ data were collected retrospectively from medical records, including initial PSA (iPSA), T stage (clinical T stage modified with MRI results when available at the initial staging), histological data (biopsies) GS topography (apex vs. middle lobe vs. base), percentage of PBC, number of anatomic levels (apex, middle lobe, and base) invaded (1 vs. 2 vs. 3), and the use of androgen deprivation therapy (ADT).
In addition, the IR-PCA patients were classified as FIR-PCA (a single IR factor and ISUP group ≤ 2 and PBC < 50%) vs. unfavorable IR (UIR) PCA (other IR patients) disease [10].
FIR patients were divided into 2 groups, those eligible for BT according to the EAU/ESTRO/SIOG guidelines, such as stage T1b-T2a N0, M0, GS 6 (ISUP grade 1) with PBC ≤ 50%, or GS 7 (3 + 4) (ISUP grade 2) with ≤ 33% of invaded biopsies, initial PSA level of ≤ 10 ng/ml, and those who were not [1].
Prostate BT was performed in all included patients with fully integrated real-time seed treatment (FIRST™) system developed by Nucletron (Elekta-Nucletron, Veenendaal, The Netherlands), that included a 3D transrectal ultrasound computer-controlled rotational system, a robotic seed delivery (SeedSelectron), and an Oncentra seeds treatment planning system [11]. The prescribed dose was 160 Gy to the prostate while minimizing the dose to the urethra and rectum, according to French and international guidelines.
A relapse was defined as a biochemical failure using ASTRO Phoenix definition or a relapse found on imaging [12]. An imaging with MRI and Cholin positron emission tomography-computed tomography (PET-CT) was recommended in case of biochemical failure, and the site of relapse was specified as local, nodal, or metastatic. Cancers occurring after BT and causes of deaths were recorded.
Median follow-up time was estimated using inverted Kaplan-Meier method. Overall survival (OS) was defined as the time from the date of BT until death resulted from any cause. Relapse-free survival (RFS) was identified as the time between the date of BT and the date of recurrence. Progression-free survival (PFS) was defined as the time between the date of BT and the date of first event (recurrence or death). Patients not known to have presented the event of interest were censored at the date of last information. Survival curves were estimated by Kaplan-Meier method and compared between sub-groups using log-rank test. Potential prognostic parameters were studied using univariate and multivariate Cox’s proportional hazards regression analysis. Statistical analyses were performed using SAS, version 9.4.
Results
Among 547 patients treated with 125I BT between 2003 and 2013, 158 patients were classified as IR-PCA according to the NCCN classification [9]. 149 IR-PCA cases were analyzed after exclusion of patients with PSA > 15 ng/ml (3 patients) and ISUP group 3 (6 patients), representing 27.2% of all patients treated during this period. Description of study population according to risk group is presented in Table 2. The median age at BT was 69 years old (range, 49-82 years old), with interquartile range (IQR) of 62-74 years. No patient received transurethral prostate resection before BT. Only 6 patients (4%) obtained short-term ADT, which started before BT, including 4 patients, who received luteinizing hormone-releasing hormone (LHRH) agonists (median, 6 months; IQR, 5.25-6 months), and 3 patients, who received non-steroid anti-androgens (median, 120 days; IQR, 75-120 days). Lots of information from pathology reports were missing to study perineural invasion and capsular effraction. MRI was available at the initial staging for 89 patients only. In total, 112 patients (75.2%) were classified as FIR-PCA, including 69 patients (46.3%) eligible to BT according to the EAU/ESTRO/SIOG guidelines. Thirty-seven patients (24.8%) presented UIR prostate cancer.
Table 2
Description of the population according to risk groups
The median follow-up was 102 months (8.5 years), ranging from 1 to 190 months (15.8 years) (IQR, 77-132 months). One patient was lost to follow-up after the scheduled 6-week visit. The follow-up ≥ 5 years for 136 patients (91.3%) was obtained.
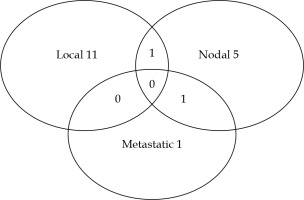
The 5-year and 10-year OS were 98% (95% CI: 94-99%) and 84% (95% CI: 75-90%), respectively. The median OS was 176.9 months (Figure 1). Of the 25 patients who deceased, only one patient died of a metastatic progression of prostate cancer, whereas 13 patients died of other cancers and 7 of intercurrent causes. No patient died of BT toxicity, and for 4 patients, the cause of death was unknown. Twenty-two (14.8%) patients developed another cancer during the follow-up after BT. The 5-year and 10-year RFSs were 91% (95% CI: 85-95%) and 77% (95% CI: 67-84%), respectively (Figure 2). Thirty patients (20.1%) experienced a relapse. The median time to a relapse was 65 months, ranging from 7 to 144 months (IQR, 43.5-96.5 months). An imaging was performed in 21 patients. The distribution of the first site of a relapse among the patients is presented in Figure 3. Two patients experienced no relapse shown on imaging and have been monitored. A local relapse was histologically proven in 8 patients (negative biopsy in 1 and biopsies not performed in 3 cases). In addition, one patient among the 9 patients, who did not benefit from an imaging exam had a proven prostate relapse after a series of biopsies.
Fig. 1
Kaplan-Meier estimate of overall survival for the entire cohort (A); for eligible FIR vs. not eligible FIR vs. UIR (B); for FIR vs. UIR (C); and for FIR eligible vs. FIR not eligible or UIR (D)
FIR – favorable intermediate-risk, eligible – eligible for brachytherapy according to the EAU guidelines, UIR – unfavorable intermediate-risk

Fig. 2
Kaplan-Meier estimate of relapse-free survival for the entire cohort (A); for eligible FIR vs. not eligible FIR vs. UIR (B); for FIR vs. UIR (C); and for FIR eligible vs. FIR not eligible or UIR (D)
FIR – favorable intermediate-risk, eligible – eligible for brachytherapy according to the EAU guidelines, UIR – unfavorable intermediate-risk

The 5-year and 10-year PFSs were 89% (95% CI: 83-94%) and 66% (95% CI: 56-75%), respectively (Figure 4).
Fig. 4
Kaplan-Meier estimate of progression-free survival for the entire cohort (A); for eligible FIR vs. not eligible FIR vs. UIR (B); for FIR vs. UIR (C); and for FIR eligible vs. FIR not eligible or UIR (D)
FIR – favorable intermediate-risk, eligible – eligible for brachytherapy according to the EAU guidelines, UIR – unfavorable intermediate-risk

There was no statistically significant difference in RFS between patients with FIR- and patients with UIR-PCA, but a trend to a difference between the 3 groups when divided the FIR group in ‘eligible’ vs. ‘non-eligible’ (p = 0.057). The difference was significant when comparing FIR-PCAs eligible to BT according to the EAU/ESTRO/SIOG guidelines (10 relapses/69) and other IR-PCA patients (20 relapses/80) (p = 0.0224). RFS curves for patients with FIR-PCA not eligible to BT according to the EAU/ESTRO/SIOG guidelines and UIR-PCAs seemed to overlap (Figure 2).
The results of univariate analysis for RFS is presented in Table 3. Failure to meet the EAU/ESTRO/SIOG criteria was significantly associated with a lower RFS, with p = 0.0267 and HR = 2.37 (95% CI: 1.10-5.08%). No multivariate analyses were conducted, as no other variables were statistically significantly associated with RFS.
Table 3
Univariate analysis associated with relapse-free survival
1 Favorable intermediate-risk according to Zumsteg classification, 2Unfavorable intermediate-risk according to Zumsteg classification, 3To brachytherapy according to European Association of Urology/European Society for Radiotherapy and Oncology/International Society of Geriatric Oncology (EAU/ESTRO/SIOG) guidelines NCCN – National Comprehensive Cancer Network
Statistically, there was no significant difference in OS and PFS between the 3 groups. However, there was a trend to a difference in PFS between the patients with FIR-PCA eligible to BT according to the EAU/ESTRO/SIOG guidelines and the other patients (p = 0.0608) (Figure 4).
Discussion
Exclusive BT, as a sole radiotherapy modality, is a well-established treatment for low-risk PCA [1, 8, 9, 13], but there are some discrepancies within the guidelines for appropriate selection criteria in the IR group of patients [1, 6, 8, 9, 13] (Table 1). Five studies have only investigated patients with IR-PCA (Table 4) [14-18].
Table 4
Series of intermediate-risk group of patients treated with brachytherapy
| Author [ref.] | Isotope | Inclusion period | Median follow-up (months) | Number of patients | Population description | Use of ADT | OS | RFS | Predictive factors |
|---|---|---|---|---|---|---|---|---|---|
| Berlin [17] | 125I | Jun, 1996-Dec, 2012 | 107.4 | 258 | NCCN IR patients | No | NA | FIR: – 5-year: 92.2% – 10-year: 88.2% UIR: – 5-year: 92.6% – 10-year: 84.8% NS | 10-year distant metastasis: – FIR 3.5% vs. UIR 10.2% (p = 0.063) 10-year prostate cancer specific mortality: – FIR 0.0% vs. UIR 2.5% (p = 0.028) |
| Frank [18] | 125I 103Pd 131Cs | 2006-2013 | 61.2 | 300 | cT1c-T2b, N0 GS 6 and PSA 10-15 ng/ml GS 7 and PSA < 10 ng/ml | No | 5-year OS: 94.9% | 5-year RFS: 92.7% | NA |
| Tran [14] | 125I | 2003-2007 | 60.0 | 615 | Only one criterion among: – stage T2b (clinical or radiological) – iPSA 10.1-20 ng/ml – GS 7 – T2b: 7% – GS 7: 63.7% – PSA 10.1-20.0: 29.3% | 18% | NA | 5-year RFS: – 87.3% (ASTRO definition) – 88.6% (Phoenix definition) | RFS: – iPSA 10.1-20 ng/ml (HR = 2.63; p = 0.0002) IR group defined by PSA 10.1-20 ng/ml and GS 6 had a 2.5-fold increased risk of biochemical failure as compared to GS 7 and PSA < 10 group |
| Herbert [15] | 125I | Jul 20, 1998-Feb 07, 2006 | 60.0 | 1,500 | GS 7: 29.3% GS 3 + 4: 24.1% GS 4 + 3: 5.1% | 94% in patients with GS 7 | NA | 5-year RFS: – 97% for patients with GS 6 – 94% for patients with GS 7 (p = 0.037) – 95% for GS 3 + 4 – 94% for GS 4 + 3 (p = 0.791) | RFS in multivariate analysis: – GS 7 (HR = 2.42; p = 0.0035) – iPSA (HR = 2.56; p = 0.0021) – ADT use (HR = 0.41; p = 0.0047) |
| Munro [16] | 125I | Mar 1995-Sep 2004 | 60.0 | 187 | T1c-T2 PSA ≤ 10 ng/ml GS 7 GS 3 + 4: 83% GS 4 + 3: 17% | 48.7% | 10-year OS: – Gleason 3 + 4: 97.3% – Gleason 4 + 3: 82.9% | 10-year RFS: 78.0% – Gleason 3 + 4: 82.1% – Gleason 4 + 3: 56.3% (p = 0.67) | RFS: D90 < 140 Gy (p = 0.08) |
| Our series | 125I | 2003-2013 | 109.0 | 149 | NCCN IR patients Exclusion of patients with PSA > 15 ng/ml and ISUP 3 | 4% | 10-year OS: 84% | 10-year RFS: 77% | RFS: Failure to meet EAU/ESTRO/SIOG criteria (p = 0.0267; HR = 2.37) |
Seventeen studies reported series of exclusive brachytherapy, including IR patients but mixed with low- [19-23] and high-risk (HR) patients [24-35], with a variable rate of patients receiving ADT up to 58.2% [19-35] (Table 5).
Table 5
Studies of mixed-risk groups of patients treated with brachytherapy
| Author [ref.] | Isotope | Inclusion period | Median follow-up (months) | Number of patients | Risk-group classification | ADT use | OS | RFS/BF | Predictive factors |
|---|---|---|---|---|---|---|---|---|---|
| Tom [24] | 125I | 1996-2017 | 48 | 2,705 | NCCN: – LR: 34.2% – FIR: 27.9% – UIR: 27.9% – HR: 9.8% | 11% (UIR 16% vs. FIR 7%; p < 0.001) | NA | 5-year BF: – LR: 4.9% – FIR: 4.3%/UIR: 17% (p < 0.001) – HR: 29.6% | Higher BF rate: – 1 UIRF (HR = 2.27; p < 0.001) – 2-3 UIRF (HR = 4.42; p < 0.001) Higher rate of distant metastasis: – 1 UIRF (HR = 2.46; p = 0.004) – 2-3 UIRF (HR = 4.76; p < 0.001) |
| Routman [19] | 125I | May, 1998- Jan, 2013 | 72 | 974 | NCCN: – IR: 29% 16.0% GS 3 + 4 4.0% GS 4 + 3 80.0% GS 3 + 3 PSA > 10 ng/ml: 10% – LR: 71% | IR 30% vs. LR 24% (p = 0.029) | LR: – 95% at 5 years – 76% at 10 years IR: – 89% at 5 years – 73% at 10 years (p = 0.22) | 10-year RFS: – LR: 90% – IR: 74% (p < 0.001) | Gleason 4 + 3 disease: – Higher rate of distant metastasis (HR = 12.4; p = 0.003) – prostate cancer-specific death (HR = 13.2; p < 0.001) |
| Chao [20] | 125I | 2004-2011 | 54 | 371 | D’Amico: – LR: 66% – IR: 33% 73% GS ≤ 6 26% GS 7 | 22.9% | 5-year OS: 96% – LR: 97% – IR: 94% (p = 0.41) | 5-year RFS: 95% – LR: 96% – IR: 92% (p = 0.09) | NS |
| Langley [25] | 125I | 1999- 2016 | 106.8 | 597 ≤ 60 yo | NICE: – LR: 53% – IR: 37% – HR: 10% | LR: BT alone IR: – ADT 6 months After 2007: – BT alone for GS 3 + 4 – HR: ADT + EBRT | 5-year OS: – LR: 100% – IR: 100% – HR: 93% 10-year OS: – LR: 98% – IR: 99% – HR: 93% (p = 0.01) | 5-year RFS: – LR: 98% – IR: 96% – HR: 92% 10-year RFS: – LR: 95% – IR: 90% – HR: 87% (p = 0.006) | NA |
| Peacock [21] | 125I | May, 2003- Dec, 2013 | 55.5 | 822 | < 2005: LR PA only > 2005: LR + IR with only one UIRF (PSA 10-20 or GS 7, or cT2b-T2c) LR: 61% | 14% | 5-year OS: 95% 10-year OS: 82% | 5-year RFS: 95% – LR: 97% – FIR: 91% 10-year RFS: 87% – LR: 91% – FIR: 72% | NA |
| Fellin [26] | 103Pd 125I | May, 1998- Dec, 2011 | 65 | 2,237 | NCCN: – LR: 66.4% – IR: 26% – HR: 1.8% | 44.5% | 3-year OS: 96.7% 5-year OS: 94% 7-year OS: 89.2% | 5-year RFS: 91.9% 7-year RFS: 88.5% Relapses: – LR: 6.3% – IR: 15.6% – HR: 24.4% (p < 0.0001) | MVA: Higher RFS in LR group (p < 0.0001) |
| Cosset [22] | 125I | Jan, 1999-Dec, 2003 | 132 | 675 | LR and selected IR (PSA 10-15 ng/ml or GS 7): – LR: 67% – IR: 33% | 58.2% | 10-year OS: 92% | 10-year RFS: 82% – LR: 87% – IR: 71% (p < 0.0001) | NS |
| Logghe [27] | 125I | Jul, 2003-Aug, 2012 | 76 | 274 | D’Amico: – LR: 63.8% – IR: 32.1% – HR: 4% | No | 5-year OS: 93.5% | 5-year RFS: – LR: 85% – IR: 70% – HR: 70% | Higher RFS: – low PSA nadir (p < 0.05) – D90 (p < 0.05) |
| Wilson [28] | 125I | Sep, 1994- Nov, 2007 | 93.6 | 176 | NCCN: – LR: 51.1% – IR: 47.7% – HR: 21.1% | 58% LR: 52.2% IR: 63.1% HR: 100% | NA | 7-year RFS: 93% 10-year RFS: 89% – LR: 96% – IR: 83% (91% FIR/74% UIR) – HR: 50% | MVA for RFS: – iPSA (p = 0.030) – HR group (p = 0.008) |
| Kittel [29] | 125I | 1996-2007 | 81.6 | 1,989 | NCCN: – LR: 61.3% – FIR: 29.8% – UIR: 4.5% – HR: 4.4% | 18.2% | 5-year OS: 93.7% – LR: 95% – FIR: 92.8% – UIR: 91.1% – HR: 84.5% 10-year OS: 76.1% – LR: 77.6% – FIR: 74.1% – UIR: 75.4% – HR: 70.6% | 5-year RFS: 91.9% - LR: 95.3% - FIR: 90% - UIR: 80.9% - HR: 67.5% 10-year RFS: 81.5% – LR: 86.7% – FIR: 79.3% – UIR: NA – HR: NA | MVA: – RFS: iPSA (p = 0.0002), GS 7 vs. 6 (p = 0.0087), GS 8-10 vs. 6 (p = 0.049), and PSA frequency (p < 0.0001) – distant metastasis-free survival: GS 7 vs. 6 (p = 0.0214) PSA frequency (p < 0.0001) – OS: GS 8-10 vs. 6 (p = 0.0053), age (p < 0.0001), Charlson score (p < 0.0001), hypertension (p = 0.0048), and smoking status (p = 0.0087) |
| Bolla [23] | 125I | Jul, 2001-Jan, 2011 | 69 | 200 | D’Amico: – LR: 83.5% – IR: 16.5% | 1.5% | 5-year OS: 96.4% 10-year OS: 89.7% | 5-year RFS: 95.6% 10-year RFS: 89.7% – LR: 89.2% – IR: 91.6% | 10-year RFS: D90 (p < 0.002) |
| Sylvester [30] | 125I | 1988-1992 | 140.4 | 215 | D’Amico: – Gleason ≤ 6: 100% – iPSA > 10 ng/ml: 18.9% – cT2b-cT2c: 10.2% | No | 15-year OS: 37.1% | 15-year RFS: 80.4% – LR: 85.9% – IR: 79.9% – HR: 62.2% | OS: iPSA > 20 ng/ml (p = 0.006) |
| Hinnen [31] | 125I | Jan, 1989-Oct, 2004 | 69 | 921 | NCCN: – LR: 25% – IR: 40% – HR: 35% | 18% | 10-year OS: – LR: 68% – IR: 64% – HR: 49% | 10-year RFS: – LR: 88% – IR: 61% – HR: 30% | MVA – RFS: IR and HR (p < 0.001), year of treatment (p < 0.001) – PCSS: HR (p < 0.001), year of treatment (p = 0.028) |
| Prada [32] | 125I | Apr, 1999- Dec, 2006 | 55 | 734 | Memorial Sloan Kettering (MSK) classification: – LR: 66.3% – IR: 29.8% – HR: 3.8% | 43% | 10-year OS: 93% | 10-year RFS: – LR: 92% – IR: 84% – HR: 65% (p < 0.001) | MVA for RFS: – GS (p = 0.003) – iPSA (p < 0.001) |
| Zelefsky [33] | 125I 103Pd | 1988-1998 | 63 | 2,693 | NCCN: – LR: 1,444 – IR: 960 – HR: 192 | No | 8-year OS: – LR: 81% – IR: 71% – HR: 63% | 8-year RFS: – LR: 74% – RI: 61% – HR: 39% (p < 0.001) | MVA for RFS: – T stage (p = 0.002) – GS (p < 0.001) – iPSA (p < 0.001) – treatment year (p = 0.001) – isotope (p = 0.004) |
| Joseph [34] | 125I | Mar, 1995- Dec, 2001 | 31 | 667 | NA | 51.9% | NA | 8.2-year RFS: 74.9% – LR: 84.3% – RI: 73.9% – HR: 52.6% (p < 0.001) | RFS: – GS (p < 0.001) – iPSA (p < 0.001) – year of implant (p < 0.001) |
| Blasko [35] | 103Pd | Jan, 1988-Dec, 1995 | 41.5 | 230 | Zelefsky: – LR: 44.8% – IR: 46.5% – HR: 8.7% | No | NA | RFS: – 83.5% at 9 years – 85.6% at 5 years At 5 years: – LR: 94% – IR: 82% – HR: 65% (p < 0.05) | MVA for RFS: – GS (p = 0.002) – iPSA (p = 0.035) |
OS – overall survival, PCSS – prostate cancer-specific survival, RFS – relapse-free survival, BF – biochemical failure, EBRT – external beam radiation therapy, UIRF – unfavorable intermediate-risk factor, LR – low-risk, IR – intermediate-risk, FIR – favorable intermediate-risk, UIR – unfavorable intermediate-risk, HR – high-risk, ADT – androgen deprivation therapy, BT – brachytherapy, iPSA – initial PSA, NA – not available, NS – not significant, MVA – multivariate analysis
– Disease risk stratification followed the guidelines issued by the National Institute for Health Care and Excellence (NICE): low-risk is defined by clinical stage T1-T2a AND a Gleason score ≤ 6 AND PSA < 10 ng/ml; intermediate-risk is T2b OR Gleason 7 OR PSA between 10-20 ng/ml; high-risk is ≥ T2c OR Gleason 8-10 OR PSA > 20 ng/ml
– The Memorial Sloan Kettering group (MSK) definition: low-risk patients are T1c or T2a, with PSA level of ≤ 10 ng/ml and Gleason score ≤ 6; intermediate-risk is T2b or PSA level 11-20 ng/ml or Gleason score ≤ 7, and high-risk: ≥ T2c or PSA level > 20 ng/ml or Gleason score ≥ 8 or 2 intermediate-risk criteria
While concerning separately the initial criteria of the disease, iPSA is an independent predictive factor in multivariate analysis for RFS [28, 29, 32-35], distant metastasis-free survival (DMFS) [29], and OS [30]. In particular, for IR patients, iPSA is an independent risk factor for a biochemical relapse [14, 15].
Gleason score is another prognostic factor associated with RFS [29, 34, 35], DMFS, and OS [29]. The effect of predominant Gleason grade (3 + 4 vs. 4 + 3) on outcomes of patients treated with exclusive BT, remains unclear. In Routman et al. study, GS 4 + 3 was a factor most strongly associated with biochemical failure (p < 0.001), incidence of distant metastasis (p = 0.003), and PCSM (p < 0.001). However, no difference in outcomes between patients with GS 3 + 3 vs. 3 + 4 was observed [19]. In a study by Burdick et al., a significant difference was found between a 5-year RFS in patients with predominant grade 3 (88%) vs. predominant grade 4 (76%) (p = 0.0231) in 127 IR and HR patients treated with BT [36]. Moreover, in Herbert et al. study, especially for IR patients, GS 7 was an independent risk factor for a biochemical relapse (p = 0.0035) in multivariate analysis, but there was no difference in estimated 5-year RFS between GS 3 + 4 vs. GS 4 + 3 (95% and 94%, respectively; p = 0.791) [14]. Munro et al. reported no significant difference between GS 3 + 4 (82.1%) and GS 4 + 3 (56.3%) (p = 0.67) groups [16].
Analyzing combined criteria, in the IR group defined by a PSA 10.1-20 ng/ml and GS 6, had a 2.5-fold increased risk of biochemical failure compared to the GS 7 and PSA < 10 ng/ml group (p = 0.0002) [14].
Up till now, no study has explored the prognostic role of PBC percentage, specifically among IR patients treated with BT alone. The validation of prognostic utility of the sub-classification of IR patients between FIR and UIR suggests a potential impact of PBC < 50% [17, 24]. In Frank et al. study, patients with GS 7, PSA < 10 ng/ml, and PBC ≤ 35% were eligible to BT alone [18]. In Kindts et al. research, PBC > 50% significantly impacted local failure (p = 0.02) [37]. Moreover, Nurani et al. observed that PBC ≥ 50% was an independent predictor of poorer RFS in patients with IR- or HR-PCA (92% vs. 81% at 5 years for patients with PBC < 50% vs. ≥ 50%; p = 0.009). However, a higher dose delivered to the prostate (D90%) minimized adverse effect of PBC ≥ 50% on biochemical failure [38]. Similarly, the percentage of PBC is probably a prognostic factor in LR patients. In two studies, with majority of LR-PCA patients, a 5-year RFS was 95% vs. 63% in patients with less versus more than 50% PBC disease (p < 0.0001), and PBC greater than median (27%) was an independent predictor of biochemical failure (p = 0.011) [39, 40].
The strength of our study is the selected homogeneous IR-PCA population, treated with BT alone (except only 4% of patients receiving short-term ADT), with a long-term follow-up. All potential prognostic factors were available (including percentage of PBC), but not D90%. Due to the retrospective nature of the present study, MRI was performed at the initial staging for only 59.7% of patients. Moreover, not all patients, who presented a relapse defined as a biochemical failure using the ASTRO Phoenix definition or a relapse identified on imaging had a histological confirmation of recurrence. Therefore, we decided to consider the most unfavorable hypothesis considering that all these patients had suffered a recurrence.
The RFS, PFS, and OS results are consistent with the existing literature regarding IR patients. As for prognostic factors, we did not find a prognostic role of isolated standard disease criterion (iPSA, T stage, GS), neither that of Zumsteg IR sub-classification, nor that of the numbers of UIR factors, as described in the literature, possibly due to a lack of power.
The incidence of the percentage of PBC alone is also not significantly associated with RFS, but it becomes meaningful when associated with other selection criteria proposed by the EAU guidelines, thus validating this European proposal.