Dear Editor,
The primary indication for enteral nutrition is the inability to receive nutrients orally. A functional gastrointestinal system with adequate length and absorption capacity is required to provide enteral nutrition. In patients who have been identified as malnourished or at risk of malnutrition, nutritional supplementation is advised. Specific indications for enteral nutrition include alternated level of consciousness, oesophageal obstruction, swallowing disorders due to neurological diseases, cancer cachexia, anorexia, intestinal fistulas of low debit, intestinal surgery, chronic bowel disease malabsorption syndrome, and pancreatitis [1].
A bezoar is a rare enteral nutrition side effect. Most reports of this complication are in the oesophagus, some in the stomach, and rarely in the small bowel. Concomitant factors of this complication are the use of drugs such as proton-pump inhibitors and sucralfate or nasogastric tube misplacement. There is no description of small bowel bezoar with concomitant use of octreotide.
Herein we present a case of an enteral nutrition bezoar in the small bowel with concomitant use of octreotide in a patient undergoing pancreatico-duodenectomy (PD) surgery.
Male, aged 61 years, with no pertinent medical history, with a recent pancreatic head tumour diagnosis, in the context of jaundice, acholia, and loss of 8 kg of body weight. PD, pancreatic-jejunal anastomosis, and duodeno-entero anastomosis were performed. Before finishing the procedure, a k-108 nasojejunal catheter was placed. In the immediate postoperative period, in the intensive care unit (ICU), it evolved with septic shock requiring mechanical ventilation, vaso-active drugs, and empiric antimicrobial agents. Enterobacter cloacae and E. faecalis were found in intraoperative cultures, and direct antimicrobial therapy was implemented.
In the immediate postoperative period, octreotide was started at 200 µg a day. Also, parenteral nutrition with a 3-chamber bag was initiated. After 48 hours, the requirement of vasoactive drugs and lactate levels decreased, and enteral nutrition was started. Polymeric and 1 kcal mL–1 and 40 gr L–1 without fibre formula was chosen and started at 21 mL h–1. On day 5 after surgery, with no feeding intolerance, the formula was changed to 1.5 kcal mL–1 and 100 g mL–1 of proteins with soluble fibre, and the infusion rate was increased to 63 mL h–1. Parenteral nutrition was stopped.
Also, on day 5 after surgery a computed tomography (CT) scan was made which showed a parietal oedema of the pancreatic anastomosis loop and perihepatic free fluid that, at the gallbladder bed, was partially loculated with isolated bubbles of pneumoperitoneum. On postoperative day 6, with the patient haemodynamically stable, 30 mg of octreotide long-acting release was given i.m. Later, on day 7, the patient showed fever, and an abdominal collection was drained percutaneously under tomographic guidance.
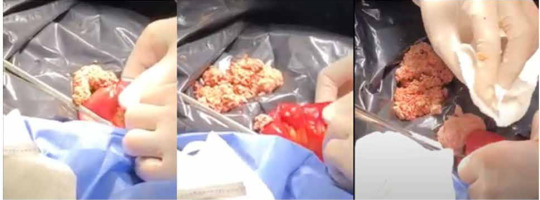
Finally, on day 8 the patient evolved with an acute abdomen, so he was admitted to the operating room. A highly dilated small intestine was found. It was observed that the cause of the intestinal obstruction was the content of the consolidated enteral diet in the form of a hard paste, such as an extensive mould. Due to this obstruction, the biliary anastomosis loop was highly dilated and obstructed, causing an anastomotic leak on the anterior surface through the suture line. Due to this great dilation of the thin, a leak was also found in the lateral border of the enteroanastomosis duodenum. An enterotomy was performed in the distal ileum, distal to the consolidated food mould, all the consolidated enteral food was extracted, and it was decided to perform the entire duodeno-entero anastomosis again; in addition, a minimal enterectomy was performed dismantling the duodeno-entero anastomosis (Figure 1). The patient returned to the ICU with refractory shock and died the next day.
FIGURE 1
Surgical removal of small bower bezoar. Consolidated enteral diet, as a hard paste, was extracted from the ileum
A bezoar is an indigestible mass that becomes stuck in the digestive system. Many substances, including fibres, the skins and seeds of fruits and vegetables, hair, medicines, and caseinate protein, can combine to form this indigestible mass. The most common place to find bezoars is the stomach, but they can be found in all the digestive tract [2].
Enteral nutrition bezoar is an uncommon complication of enteral nutrition. Most of the cases presented in the literature are related to nasogastric tube misplacement (Table 1). A case of nasogastric tube misplacement and oesophageal bezoar was reported in a 20-year-old male with meningoencephalitis and prolonged enteral feeding. It was resolved by removing the tube and washing the oesophagus with bicarbonate solution [3]. The use of pancreatic enzymes for the dissolution of the bezoar was also described [4, 5]. In another case, a 69-year-old woman was under mechanical ventilation after cardiac arrest, receiving enteral nutrition by nasogastric tube; after misplacement of the tube, the reinsertion was difficult [6]. An endoscopy was performed, and an oesophageal bezoar was diagnosed. Due to the terminal condition of the patient, it was not removed and a jejunostomy was performed [6]. Also, the case of a 74-year-old with myocardial infarction and chronic obstructive pulmonary disease with long hospital stay was reported, in whom, after tube misplacement, reinsertion was difficult. Endoscopic diagnosis of an oesophageal bezoar was made, and mechanical endoscopic treatment was performed [7]. In an instance involving an 80-year-old woman with chronic mechanical ventilation in a long-term facility and enteral nutrition with a ready-to-use formula and protein supplement, after tube misplacement, reinsertion was again difficult [8]. Endoscopic diagnosis of an 18-cm oesophageal bezoar was made, and endoscopic fragmentation was performed, and the rest was pushed to the stomach [8]. In all the cases, after the tube misplacement, the reinsertion was difficult, being this an early sign that could alert physicians. But in a case report of an oesophageal bezoar, the solidification only encircled the tube, and only the change of the tube for a new one was needed [9]. Table 1 shows the oesophageal bezoars reported in the literature. Finally, Caldeira et al. [10] published a retrospective study with 1003 ICU patients with enteral nutrition, and 9 of them had oesophageal impactation of enteral nutrition, it was also shown that 7 of them had oesophageal reflux risk factors. Seven individuals responded favourably to the endoscopic therapy. Also, it was observed that the average time of impact of enteral feeding was 12 days after the beginning of enteral feeding [10].
TABLE 1
Case reports of oesophageal bezoars and different treatment approaches
| Author, year of publication [Ref.] | Patient condition | Concomitant medication | Length of enteral feeding (EN) before bezoar | Treatment |
|---|---|---|---|---|
| Tawfic, 2012 [3] | 20-year-old man with meningoencephalitis | Piperacillin-tazobactam, acyclovir, sodium valproate, levetiracetam, omeprazole | Two and a half months | Endoscopic washing with bicarbonate solution |
| Gil-Almagro, 2015 [4] | 66-year-old man in the intensive care unit for an extended period | Opioids intravenous | Three months | Pancreatic enzymes |
| Bouwyn, 2011 [12] | 67-year-old patient with septic shock | Lactulose | Seven days | Pancreatic enzymes |
| Degheili, 2017 [6] | 69-year-old woman with cardiac arrest | Not reported | Not reported | None |
| Krupp, 1995 [7] | 74-year-old man with myocardial infarction | Aluminium hydroxide gel and sucralfate | 12 days | Flexible and rigid endoscopic fragmentation and pushing to the stomach |
| Marcus, 2010 [8] | 80-year-old woman with chronic mechanical ventilation | Furosemide, levothyroxine, omeprazole, prednisone, carbidopa-levodopa, amiodarone and subcutaneous insulin | Not reported | Endoscopic fragmentation and pushing to the stomach |
| García-Luna, 1997 [9] | 40-year-old woman with subarachnoid hemorrhage | Sucralfate, B complex vitamins, iron and magnesium salts, phenytoin, phenobarbital | 110 days of mechanical ventilation; length of EN not reported | Change of tube |
| 61-year-old man | Sucralfate, vitamins B and C, iron | 42 days after admission; length of EN not reported | Endoscopic removal failed – total parenteral nutrition | |
| 66-year-old man with 35% body surface burn | Sucralfate, lactulose, vitamin complexes | Seven days | Replacement of tube, and 3 days later PEG | |
| Cremer, 1996 [28] | 68-year-old man with myasthenia gravis | Pyridostigmine, prednisone, metoclopramide, trimethoprim-sulfamethoxazole | Seven months | Not reported |
| Katsanos, 2010 [29] | Patient under mechanical ventilation with trauma and abdominal compartment syndrome with surgical treatment; no other data reported | PPI | Not reported | Endoscopic with a Roth net and sprayed with a mixture of Gastrografin and N-acetylcysteine |
| Patient with stroke under mechanical ventilation; no other data reported | PPI | Not reported | Endoscopic with a Roth net and sprayed with a mixture of Gastrografin and N-acetylcysteine |
Also, a non-oesophageal bezoar was reported in the case of gastric bezoar in a 58-year-old woman after cardiac arrest receiving enteral nutrition on day 28. The diagnosis was made with an abdominal X-ray that showed the stomach filled with calcifications [11]. A case of a diffuse bezoar in the stomach and small bowel was reported in a patient with legionella pneumonia with adult respiratory distress syndrome [12]. In this case the bezoar was attributed to the initiation of enteral nutrition during paralytic ileus after surgical treatment of alithiasic cholecystitis, the use of opioids, and the overlap between enteral nutrition and norepinephrine [12]. Also, a small bowel bezoar was reported in a 67-year-old male patient in the postoperative period after gastrectomy with a Roux-en-Y reconstruction and cholecystectomy, with enteral nutrition through a jejunostomy [13]. The patient needed surgery, denatured enteral formula was found proximal to the jejunostomy tube, and it was drained with a longitudinal incision of the jejunum [13]. Similarly, a 36-year-old pregnant woman with ovarian cancer needed a total gastrectomy with a Roux-en-Y reconstruction and cholecystectomy, and a jejunostomy tube was inserted [13]. Other places of enteral nutrition bezoar were reported, e.g. the caecum [14]. Table 2 shows cases of enteral nutrition non-oesophagus bezoars.
TABLE 2
Case reports of enteral nutrition bezoars in locations other than the oesophagus
| Author and year of publication | Patient condition | Location of bezoar | Concomitant medication | Length of enteral feeding (EN) before bezoar | Treatment |
|---|---|---|---|---|---|
| Enomoto, 2016 [11] | 58-year-old woman with cardiac arrest | Gastric | Not reported | 21 days | Coca-Cola® through nasogastric tube |
| Bouwyn, 2012 [12] | 65-year-old man with pneumonia | Gastric and small bowel | Not reported | 46 days | Surgical |
| Dedes, 2006 [13] | 67-year-old man with gastrectomy and cholecystectomy | Small bowel | Not reported | 7 days | Surgical |
| 36-year-old woman with ovarian cancer and gastrectomy | Small bowel | Not reported | 4 days | Surgical | |
| Siddens, 2020 [15] | 70-year-old woman with pancreatic cancer and pancreaticoduodenectomy | Small bowel | Laxatives | 5 days | Surgical |
| O’Neil, 1996 [30] | 79-year-old with gastric adenocarcinoma and gastrectomy | Small bowel | Not reported | 7 days | Papain through the tube every 6 hours |
A case of a small bowel bezoar with enteral feeding in a 70-year-old woman with pancreatic cancer who required a pancreaticoduodenectomy was published in 2020, like the present case. The diagnosis was made on day 5. The only concomitant medications reported were laxatives, and she was not under vasopressors. The patient needed surgery and was discharged home alive after 37 days [15]. The difference between this and the current case is that the patient was under vasopressors, with mechanical ventilation, and was receiving octreotide.
Pancreaticoduodenectomy, a complex abdominal surgery, is indicated in malignant tumours of pancreatic head, ampulla, periampullary, distal bile duct, benign tumours, trauma of pancreatic head and duodenum, as well as non-neoplastic conditions such as chronic pancreatitis [16–20]. Due to the severity of the disease and the nutritional status in the postoperative period, most of the patients require nutritional support with parenteral nutrition, enteral nutrition, or both [21]. The mortality rate of PD is below 5% in specialized centres, but it has high postoperative morbidity rates of up to 60%. Major postoperative complications include bile leak, pancreatic fistula and delayed gastric emptying, postoperative haemorrhage requiring blood transfusion or reopening, abdominal abscess, wound infection, or dehiscence [16, 17, 19, 22, 23].
The rate of pancreatic fistula varies greatly between studies, ranging from 5 to 35% [16, 24, 25]. Most studies demonstrate that even high-output fistulas (those that produce more than 200 mL per day) can spontaneously shut with conservative therapy, while the closure rate is noticeably lower than that of low-output fistulas [16, 24, 25]. Conservative treatment consists of electrolyte replacement, fluid balance, parenteral nutritional support, if required, antibiotics in patients with sepsis signs or local inflammation, and drainage of intra-abdominal collections [17]. Furthermore, the use of somatostatin has been proposed because it increases the net absorption of water and electrolytes while inhibiting pancreatic exocrine, biliary, and small bowel discharges [24–26]. Despite its benefits, somatostatin and its analogues have been shown in numerous studies to speed up fistula closure and decrease the discharge of digestive fistulas, but there is no solid evidence for higher closure rates compared with standard treatments [24–26]. The short half-life of somatostatin has led to the development of several analogues. Due to octreotide’s 120-minute half-life, intermittent subcutaneous dose regimens are possible and were employed in the actual case. Colonic pharmaco-bezoar related with octreotide use was reported in a 79-year-old patient on the 14th day after cephalic pancreaticoduodenectomy with concomitant use of this drug [27]. Although it is not an enteral nutrition bezoar, this case is useful to exemplify a complication related to the use of octreotide.
As has been shown, enteral nutrition bezoar is an unusual complication of enteral nutrition. Several factors can increase the chance to generate it, such as nasogastric tube misplacement or concomitant use of drugs like sucralfate, opiates, norepinephrine, or, as in this case, somatostatin analogues. The use of new antacids has reduced the use of sucralfate, and multimodal analgesia has reduced the use of opiates. The aim of treatment is to remove the bezoar, because it may result gastric outlet obstruction, ileus, ulcerations due to pressure necrosis, and subsequent gastrointestinal bleeding. Depending on the location of the bezoar, different strategies for removal can be implemented. As presented before, in oesophageal bezoars, endoscopic fragmentation and pushing to the stomach can be tried, and in small bowel bezoars, surgery is the solution. Also, other strategies have been described, including effervescent liquids (Coca-Cola®), N-acetylcysteine, Gastrografin spray, and pancreatic enzymes.




