Video-assisted thoracoscopy (VATS) has become a standard approach in the surgical care of patients undergoing thoracic surgery [1]. Despite advances in surgical modalities, the incidence of chronic pain (CP) after thoracoscopy is still very high (25%) [2]. Furthermore, with the upcoming enhanced recovery after surgery (ERAS) guidelines, regional anaesthesia is paramount to reduce postoperative opioid use and postoperative complications [3, 4]. Thoracic paravertebral block (TPB) and thoracic epidural (TE) are the gold standards in regional anaesthesia techniques to control acute pain after classic thoracic surgery (open thoracotomy) [5]. However, TE and TPB are only used in 10% and 50% of cases, respectively [6, 7] among patients undergoing VATS. Therefore, there is an increased interest in an alternative regional anaesthesia technique for postoperative pain management.
The erector spinae plane block (ESPB) was first described in 2016 to treat chronic thoracic neuropathic pain and was later successfully used in VATS surgery for acute pain control [8–10]. Therefore, we performed this prospective observational study to investigate whether there is an association between ESPB and incidence of chronic neuropathic pain (CNP) and quality of life (QoL) after surgery. The a priori primary outcome was the incidence of CNP three months postoperatively (POP) in patients undergoing VATS surgery with ESPB as the regional anaesthesia technique. The secondary outcome consisted of pain control at the post-anaesthesia care unit (PACU), 12- and 24-hour POP and QoL reported up to three months after surgery. We hypothesised that patients with ESPB would have a low incidence of acute and CNP and report a good QoL.
METHODS
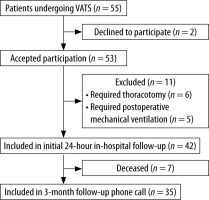
The study was a single-centre prospective, longitudinal, observational cohort study approved by the institutional review board of Hospital Universitario San Ignacio on January 2019 (FMCIE- 0043-19). It was registered at ClinicalTrials.gov (ID: NCT04737902). Patients older than 18 years who underwent VATS and required at least one day of hospital stay after surgery were recruited from January to April 2020. Subjects with cognitive limitation to answer the questionnaires, who required mechanical ventilation or conversion to thoracotomy, or declined consent were excluded. Written informed consent was obtained from all participants. This article adheres to the applicable STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines [11].
Study protocol
Study subjects underwent anaesthesia and surgery without a change in their routine care. All patients under general anaesthesia were intubated with a double-lumen endotracheal tube. At the end of the surgery, before extubation, ESPB was performed for acute pain control following our institutional protocol for perioperative care.
Unilateral ESP block was performed between T3 and T7 levels depending on the thoracic surgical incision following the technique described by Forero et al. [8]. Briefly, the patient was placed in the lateral decubitus position, under sterile conditions. A high frequency (6–15 MHz) linear-array transducer (Sonosite Edge II, Bothell, USA) was placed in a longitudinal parasagittal orientation 2 cm from the posterior midline to visualise the tips of the transverse processes deep to the erector spinae muscle (ESM). A 21 G, 50 mm or 100 mm needle (Pajunk UniPlex NanoLine; Germany) was advanced in-plane with the ultrasound beam. The needle tip was directed to the plane between the transverse process and the posterior fascia of the ESM. Correct needle tip location was confirmed by ultrasound visualization of linear fluid spread in the fascial plane. Injection of 0.5 to 1 mL of bupivacaine 0.5% with epinephrine 5 µg mL-1 was performed; then, a total of 20–30 mL of the same local anaesthetic was administered.
Data collection and endpoint definition
We collected data on age and gender, ASA physi-cal status, type of surgery (urgent or elective), clinical diagnosis (infection, metastasis, primary cancer, and systemic inflammatory disease), surgical procedure (biopsy, lobectomy, and pleurectomy), surgical duration, and technical difficulty to perform ESPB reported by the anaesthesiologist medical staff.
The primary and secondary outcomes were defined a priori. For the primary outcome, patients were contacted by phone at three months after surgery to collect information about CNP using the DN4 (neuropathic pain diagnostic questionnaire) [12]. For our secondary outcomes, numerical rating pain scale (NRS) scores (0–10, 0 = no pain, 10 = worst pain imaginable), details of the analgesic consumption (opioids and non-opioids) in the operating room and PACU, 12 and 24 hours after the end of surgery were collected; all opioids were converted to morphine milligram equivalents for analysis [13]. Dynamic pain was defined as pain induced by a deep breath, cough, movement, or incentive spirometer. Adequate pain control was defined following the definition of Cepeda et al. [14] where a decrease of 2.4 and 3.5 points on the NRS corresponded to “much improvement” and “very much improvement” respectively. We also assessed the QoL using the EuroQoL-5 dimensions (EQ-5D) questionnaire and analysed it according to the recommendations of Herdman et al. [15]. The EQ-5D “thermometer” gives information about QoL on a continuous scale; its transformation into a categorical rating scale has been validated to help with its interpretation [16], with scores over 80 correlating with “very good” QoL.
Statistical analysis
This study was designed as a pilot study assessing feasibility and recruitment rate. Measures of central tendency and dispersion were calculated for continuous variables (interquartile range [IQR]), and categorical variables were presented as frequencies and proportions. We calculated the 95% confidence interval (CI) for each result. Due to the non-normal data distribution, the analysis was performed using non-parametric statistics.
To compare the median basal NRS value at the PACU, 12, 24 hours, and three months after surgery, the Friedman test was used. To assess whether there is an association between NRS scores, ade-quate pain control, CNP presence, and the study’s independent variables, we calculated the contingency coefficient for dichotomous and polychotomous nominal variables. The eta coefficient was used to determine the strength of association between the categorical variables and interval level variables. Fisher’s exact test was used to determine the association between dichotomous variables. All statistical analysis was undertaken using SPSS Statistics v.22.0 (IBM Corp., NY, USA). Statistical significance was established as a P-value ≤ 0.05.
RESULTS
Fifty-five patients were approached to parti-cipate in the study (Figure 1). Forty-two patients were enrolled and completed initial PACU, 12, and 24-hour in-hospital assessment. Our cohort was a predominantly oncologic population, with 78.6% of patients undergoing VATS due to thoracic malignancy. The median length of hospital stay was 48 hours (IQR 25.5–72.0 hours) (Table 1).
TABLE 1
Patients’ baseline characteristics
Final follow-up was conducted until April 2020, three months after inclusion of the last patient. We obtained information for 35 patients, as 7 of them (16.7%) died during these three months due to the progression of their oncological disease.
Three (7.1%) of the 42 ESPB performed in our cohort were deemed technically challenging, with no reported complications. Average NRS score for static pain at PACU arrival was 5.74, with a median of 4 mg of intravenous (IV) morphine equivalents (IQR 3.0–7.0) needed in the PACU to achieve adequate pain control. Table 2 depicts the immediate postoperative systemic multimodal analgesic mana-gement in our cohort.
TABLE 2
Perioperative multimodal analgesic management
We found a median IV morphine-equivalents consumption of 5.5 mg in the first 24 hours of in-hospital stay (PACU excluded). Median NRS score was 3 for 12-hour and 24-hour pain at rest, respectively. “Much improvement” was noted in 52.38% of our patients (95% CI: 36.42–68.00) at 12-hour POP and in 57.14% (95% CI: 40.96–72.28) at 24-hour POP. Conversely, we found minor pain relief in the dynamic assessment: a median NRS score of 6 at 12-hour and 24-hour POP and “much improvement” in 26.19% (95% CI: 13.86–42.04) at 12-hour POP and 23.81% (95% CI: 12.05–39.45) at 24-hour POP (Table 3).
TABLE 3
Postoperative numerical rating pain scale (NRS) scores*
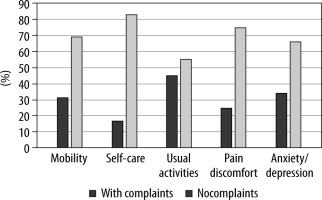
At three months POP, no patient reported CNP using the DN4 questionnaire. QoL three months after surgery (Figure 2), as assessed by the EQ-5D instrument, was reported as “very good” by 54.3% of patients [16]. Interestingly, most QoL complaints in our cohort were reported as minor problems related to everyday activities (i.e., work, study, housework, family, or leisure activities); and 74.3% of patients reported no pain or discomfort. No statistically significant association was found between perioperative management variables or in-hospital NRS scores and QoL three months after surgery.
DISCUSSION
The main finding of our pilot study is the low incidence of CNP after VATS with ESPB performed as a primary analgesic technique. None of our patients had CNP, contrasting with the CNP incidence worldwide after VATS ranging from 15 to 36% despite different analgesic techniques used (TE, TPB, patient-controlled analgesia, among others) [2, 4, 9, 10]. Also, the recruitment rate was adequate, and ESPB was feasible in all investigated patients without complications.
Furthermore, the severity of average acute pain after thoracic surgery is associated with subsequent CP; each point of increase in the NRS score increases the probability of developing CP by 1.3 times [2]. ESPB has been used for CNP treatment (or prevention) [8]; in an observational study, its use was associated with a lower incidence of CP eight weeks after VATS when compared with an intercostal block (10.6 % vs. 35.4%, respectively; P = 0.008) [17]. ESPB has been used in other types of surgery within the chest cavity; for example, a study by Wiech et al. [18] investigated patients undergoing cardiac surgery receiving ESPB, with better acute and chronic pain control after surgery, suggesting that it may decrease the risk of developing CP up to six months after surgery. Other regional techniques (e.g., TPB) have also decreased the intensity of acute and chronic pain (up to 6 months) after open thoracotomy [19]. Therefore, our findings raise the question (and provide pilot data) whether ESPB can diminish the incidence of CP and CNP after VATS.
Interestingly, dynamic pain in our cohort showed minimal improvement during hospital stay compared to pain at rest. Similar findings could be explained by the study of Taketa et al. [9], where NRS on movement was higher than NRS at rest; however, they had lower dynamic NRS scores than our cohort, probably secondary to the use of an ESP catheter. The meta-analysis of Cai et al. [20] found that ESPB was associated with lower pain scores at rest and during movement at 12 h and 24 h after surgery. Nonetheless, these results should be interpreted with caution as they had substantial heterogeneity and included small sample studies. Additio-nally, the difference between pain relief at rest and on movement can be secondary to the insufficient distribution of a single-shot ESPB to cover every dermatome involved in VATS and chest tube; thus, a randomised study is needed to elucidate it.
Although the QoL was “very good” in half of our cohort, patients with lower scores mainly complained about the limitations in “usual activities” and not in the “pain/discomfort” category. These findings may be partially explained by the fact that our studied population consisted of predominantly oncological patients with the so-called whole-person concerns associated with malignant disease [21].
In this study, patients who underwent VATS with an ESPB had adequate pain relief and minimal IV morphine-equivalent consumption. These findings contrast with those reported by Duclos et al. [22] (23 mg; IQR 16.5–39.0) or by Kamalanathan et al. [23] with early paravertebral bupivacaine (34 mg; IQR 7.3–105.0) or after late paravertebral bupivacaine (40.7 mg; IQR 3–91). This difference can be associated with the analgesic technique, the ethnicity influence in the morphine consumption described by Cepeda et al. [24] or the low prevalence of younger patients (< 50 years old), who have an increased risk of moderate-severe postoperative pain after VATS surgery (OR, 0.96; 95% CI: 0.95–0.98, P < 0.001) [25].
Traditionally, TE and TPB have been described as the gold standard in pain management after thoracic surgery [26]. However, the effectiveness of TE may be limited by a considerable failure rate, the risk of urinary retention and a greater incidence of postoperative hypotension [5], and TPB remains a technically challenging procedure with a steep learning curve and risk of bradycardia and hypotension, local anaesthetic toxicity, and pneumothorax, limiting its use [6, 7, 27]. Using fascial plain blocks can eliminate many of these limitations and expand available pain management options after thoracic surgery. ESPB is non-inferior to TPB for postoperative analgesia after VATS surgery and was found to be an effective strategy for postoperative analgesia in this surgery [9, 10] with substantial reproducibility and a smaller risk of complications [28].
We are aware of several limitations of our study. First, the concept of optimal pain management after thoracic surgery is the centre of an ongoing debate, and the clinical significance of defining it by reductions in the NRS score has been subjected to a comprehensive critique [14, 29]. Other measures of pain relief can provide a more patient-centred approach to postoperative pain management [29]. However, reduction of the NRS score has been measured in several studies, and it can be used as a benchmark against which our findings can be compared. Second, it is a single-centre observational study, and its relatively small sample size may have been underpowered to detect some clinically significant associations. On the other hand, our results highlight the need for larger-scale multicentre studies to delineate the potential role of ESPB to diminish the incidence of CNP or its influence on the quality of life. Third, we did not obtain a baseline (before surgery) QoL assessment, which prevented us from evaluating the potential impact of VATS in that domain, or whether surgery exacerbated specific health complaints measured by EQ-5D. Its strengths include the longitudinal study design, which allowed us to follow the patients’ perioperative care, describe CNP incidence, and assess these patients’ QoL after VATS with an ESPB.