Introduction
Oral carcinoma is the sixth most common malignancy worldwide [1]. Out of all new cases of oral carcinoma reported worldwide in 2012, 2/3 of the tumours were reported in developing countries, and South and Southeast Asia and some countries in southern Europe had the highest incidence. This carcinoma causes almost 145,328 deaths annually worldwide [2]. Oral squamous cell carcinoma (OSCC) – the most prevalent oral carcinoma – is an important health problem in the world, and around 600,000 cases are diagnosed annually [3]. Therefore, the prevalence and clinical pattern of OSCC change considerably depending on the geographical location where it is diagnosed [2]. Unfortunately, despite the advanced methods in surgery, radiotherapy, and chemotherapy, the mortality of OSCC is high [4]. When oral carcinoma is determined early in stages I–II, the patients’ survival increases from 60% to 80% [5]. A suppressed immune system is an established phenomenon in OSCC patients and can include changes in cytokines and the balance of immune cells [6]. Development of molecular biology has shown that matrix metalloproteinases (MMPs) play a pivotal role in the cancer progression, invasion, and angiogenesis [7]. Also, they impact the OSCC progression either by directly changing the extracellular environment or indirectly via beginning vascular regression [8, 9]. The MMP family includes diverse substrates [10]. They are a large family of zinc-dependent endopeptidase and are critical to the physiologic degradation of the extracellular matrix [11]. MMPs are made up of a prodomain, a catalytic domain, a hinge region, and a hemopexin domain that have six separated groups [12]. Therefore, the measurement of some MMPs in OSCC patients may be examined for the diagnosis and prognosis of this disease [13].
Aim
This meta-analysis was conducted to determine the difference between the OSCC patients and the healthy controls in the serum and salivary MMP levels.
Material and methods
Study protocol
The protocol of the present meta-analysis was followed based on PRISMA guidelines [14].
Focused question
Are serum and salivary MMP levels (intervention) related to OSCC (outcome) compared to controls (comparison) in people (population)?
Search strategy and study selection
The electronic databases of Scopus, Web of Science, PubMed, and Cochrane Library were systematically searched to identify the relevant articles up to March 2019 without language restriction. The search terms (“matrix metalloproteinase” OR “MMP”) AND (“OSCC” OR “oral squamous cell carcinoma” OR “oral SCC” OR “oral cancer” OR “oral carcinoma”) AND (“serum” OR “saliva” OR “salivary” OR “blood”) were used in the databases to find the relevant articles. In addition, the citations in the systematic review/review articles on the topic were checked and reviewed.
Two authors (M.S. and F.R.) conducted the search and reviewed all articles retrieved from the search. In the first stage, duplicate articles were excluded. In the second stage, the titles and abstracts of the remaining articles were screened to exclude irrelevant articles. In the third stage, full texts of articles that met the eligibility criteria were assessed, among which some full texts were excluded with reasons. At last, the remaining full texts were included in the systematic review and then in the meta-analysis. The disagreements about the extracted data between both authors were resolved by a discussion.
Eligibility criteria
Inclusion criteria: 1) case-control studies reporting salivary/serum MMP levels and including OSCC patients with any stage and healthy controls and 2) diagnosis of OSCC histologically and/or clinically. Exclusion criteria: commentaries, letters to the editor, editorials, case reports, reviews/systematic reviews, conference abstracts, book chapters, and studies with irrelevant data.
Data extraction
Some data were retrieved from each study, including the first author’s name, publication year, number of patients and controls, mean age and male/female ratio of patients and controls, measurement method of serum and salivary MMP levels, type of measured sample, and type of measured MMP. One author (M.S.) did data extraction and another author (F.R.) re-checked it.
Quality assessment
Critical evaluation of the included studies was organized using the quality assessment tool of the Newcastle-Ottawa Scale (NOS), with a maximum score of 9 for each study [15]. This tool was used to assess the risk of bias in individual studies and to grade the study quality as high (≥ 7), fair (4–7), or low (< 4). The quality assessment was conducted by one author (M.S.).
Statistical analysis
The standard mean difference (SMD) and its 95% confidence interval (CI) were obtained for each study by Review Manager 5.3 (RevMan 5.3, Cochrane Collaboration) to explain the difference between the OSCC patients and the healthy controls in the salivary and serum MMP levels (p-value less than 0.05 was considered statistically significant). The degree of heterogeneity was estimated using the I2 statistic among the studies. P < 0.1 (I2 > 50%) showed a significant heterogeneity, following which the random-effects model was conducted. The analysis of funnel plot was done by the Comprehensive Meta-Analysis version 2.0 (CMA 2.0) software using both Egger’s and Begg’s tests with a p < 0.05 (2-tailed) considered to be a significant publication bias. We used the removal of one study and cumulative analysis to assess the stability/consistency of the pooled results. The unit of salivary and serum levels of MMPs was ng/ml in the present meta-analysis. If there was median (interquartile range) in the studies, we converted it to mean ± SD by formula [16].
Results
Study selection
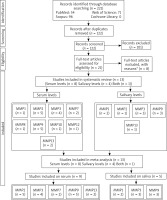
Figure 1 shows the process of the literature search. Out of 221 studies retrieved from the databases. After removing duplicate and irrelevant studies, 21 studies were assessed based on their full texts. Out of 21 studies, 9 studies were excluded for reasons given (2 had no control group, 1 was a review, 1 was a book chapter, 2 had irrelevant data, 2 included laryngeal cancers, and 1 reported a polymorphism of MMP). After that, 13 studies were included in systematic review, from which 9 studies were reported on serum and 5 studies on salivary levels. The number of studies reporting MMP levels in serum or saliva is shown in Figure 1. At last, 13 studies (9 on serum and 5 on salivary levels) were analysed in the meta-analysis (serum MMPs 2, 3, 7, 9, and 13 and salivary MMPs 1, 3, 9).
Study characteristics
The characteristics of the included case-control studies are presented in Table 1. Out of thirteen studies included in the meta-analysis, five studies were from Iran [4, 17–20], two from China [6, 21], two from Germany [22, 23], one from Korea [24], one from Taiwan [25], one from Egypt [26], and one from Pakistan [27]. The studies had been published from 2008 to 2019. Four studies [4, 20, 22, 25] reported MMP levels on saliva and eight studies [6, 17–19, 21, 23, 24, 27] on serum and one study [26] included both samples. The measurement method of saliva or serum levels of MMPs was ELISA except for one study [25] that was mass spectrometry. Among all studies, nine studies included the OSCC patients with stages I to IV [6, 17–19, 21–25], two studies stages I and II [4, 26], and two studies [20, 27] did not report. The rest of data, including the number, mean age, and male/female ratio of OSCC patients and controls are shown in Table 1. Two studies [22, 27] reported median (interquartile range), which was converted to mean ± SD.
Table 1
Characteristics of included case-control studies
| First author, publication year | Country | No. of patients | No. of controls | Patients (male/female) | Controls (male/female) | Patients (mean age) [years] | Controls (mean age) [years] | Method | Sample | Measured MMP | Stage of OSCC |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lee, 2008 [24] | Korea | 37 | 20 | NA | NA | 59.1 | NA | ELISA | Serum | MMP2, MMP9 | I–IV |
| Tadbir, 2012 [17] | Iran | 45 | 45 | 22/23 | 22/23 | 57.0 | 56.6 | ELISA | Serum | MMP3 | I–IV |
| Chang, 2013 [21] | China | 151 | 111 | 142/9 | 101/10 | 52.5 | 48.3 | ELISA | Serum | MMP2, MMP9 | I–IV |
| Andisheh-Tadbir, 2014 [18] | Iran | 45 | 45 | 22/23 | 22/23 | 57 | 56.6 | ELISA | Serum | MMP3 | I–IV |
| Lotfi, 2015 [19] | Iran | 20 | 20 | 11/9 | 10/10 | 61.3 | 53.9 | ELISA | Serum | MMP2, MMP9 | I–IV |
| Jiang, 2016 [6] | China | 204 | 212 | 157/47 | 155/57 | 57.3 | 56.6 | ELISA | Serum | MMP7 | I–IV |
| Yu, 2016 [25] | Taiwan | 131 | 96 | 129/2 | 96/0 | 52.5 | 48.8 | LC-MRM- MS | Saliva | MMP1, MMP3, MMP9 | I–IV |
| Ghallab, 2017 [26] | Egypt | 15 | 15 | 6/9 | 6/9 | 47.7 | 43.3 | ELISA | Serum, saliva | MMP9 | I, II |
| Nosratzehi, 2017 [20] | Iran | 30 | 30 | NA | NA | NA | NA | ELISA | Saliva | MMP1, MMP2 MMP3, MMP13 | NA |
| Peisker, 2017 [22] | Germany | 30 | 30 | 14/16 | 18/12 | 65.0 | 60.7 | ELISA | Saliva | MMP9 | I–IV |
| Schiegnitz, 2017 [23] | Germany | 81 | 49 | NA | NA | 68.0 | 58.0 | ELISA | Serum | MMP2, MMP3, MMP13 | I–IV |
| Lee, 2018 [24] | Iran | 15 | 30 | 9/6 | NA | NA | NA | ELISA | Saliva | MMP3 | I, II |
| Choudhry, 2019 [27] | Pakistan | 38 | 38 | 26/12 | 23/15 | 50.9 | 50.8 | ELISA | Serum | MMP1, MMP2 MMP3, MMP7 MMP8, MMP9 MMP10, MP12, MMP13 | NA |
Meta-analysis reports
Serum levels
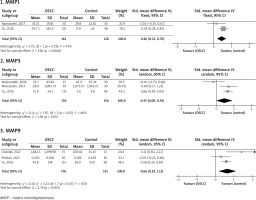
Figure 2 shows serum levels of MMPs 2, 3, 7, 9, and 13 in OSCC and healthy controls. The serum MMP2 level was evaluated in five studies [19, 21, 23, 24, 27] with a total of 327 OSCC patients and 238 controls, the pooled SMD of which was found to be 0.49 ng/ml (95% CI: –0.47, 1.45; p = 0.32; I2 = 96%, Ph < 0.00001). The serum MMP3 level of 209 patients and 177 controls in four studies [17, 18, 23, 27] reached a pooled SMD of 0.44 (95% CI: –0.03, 0.91; p = 0.06; I2 = 80%, Ph = 0.002). The serum level of MMP7 was reported in two studies [6, 27] on 242 patients and 250 controls, the pooled SMD of which was 0.78 (95% CI: 0.15, 1.41; p = 0.02; I2 = 84%, Ph = 0.01). A total of 261 patients and 204 controls were included in five studies [19, 21, 24, 26, 27] for analysis of the serum level of MMP9 with a pooled SMD of 1.18 (95% CI: 0.51, 1.84; p = 0.0005; I2 = 88%, Ph < 0.00001). In addition, 119 patients and 87 controls were included in two studies [23, 27] showing the serum MMP13 level, the pooled SMD of which was obtained to be 0.42 (95% CI: –0.05, 0.90; p = 0.08; I2 = 63%, Ph = 0.10). Therefore, among the analyses of serum MMP levels, just the serum MMPs 7 and 9 levels were significantly higher in the OSCC patients than the healthy controls.
Salivary levels
Figure 3 illustrates the pooled analysis of the salivary levels of MMPs in the OSCC patients compared to the healthy controls. Two studies [25, 26] including a total of 161 patients and 126 controls reported that the pooled SMD of the salivary MMP1 level was 0.46 (95% CI: 0.22, 0.70; p = 0.0001; I2 = 0.43%, Ph = 0.19). Three studies [4, 20, 25] included a total of 176 patients and 156 controls and reported the pooled SMD of 0.45 (95% CI: 0.00, 0.91; p = 0.05; I2 = 66%, Ph = 0.05). In addition, three studies included a total of 176 patients and 141 controls [22, 25, 26] and showed that the pooled SMD for the salivary MMP9 level was 0.66 (95% CI: 0.19, 1.12; p = 0.005; I2 = 62%, Ph = 0.07). Therefore, analyses of the salivary levels showed that the MMPs 1 and 9 levels were significantly higher in the OSCC patients than the controls.
Quality assessment
The results of quality assessment showed that the mean square of all included studies was 7.3 (Table 2). Eight studies had high quality (score ≥ 7) and five studies had fair quality (score of 4–7).
Table 2
Quality assessment of the included case-control studies
| First author, publication year | Selection | Comparability# | Exposure | Total points |
|---|---|---|---|---|
| Lee, 2008 [24] | ** | – | *** | 5 |
| Tadbir, 2012 [17] | **** | ** | *** | 9 |
| Chang, 2013 [21] | *** | ** | *** | 8 |
| Andisheh-Tadbir, 2014 [18] | **** | ** | *** | 9 |
| Lotfi, 2015 [19] | *** | ** | *** | 8 |
| Jiang, 2016 [6] | *** | ** | *** | 8 |
| Yu, 2016 [25] | ** | * | *** | 6 |
| Ghallab, 2017 [26] | **** | ** | *** | 9 |
| Nosratzehi, 2017 [20] | ** | – | *** | 5 |
| Peisker, 2017 [22] | *** | ** | *** | 8 |
| Schiegnitz, 2017 [23] | ** | * | *** | 6 |
| Lee, 2018 [24] | *** | – | *** | 6 |
| Choudhry, 2019 [27] | *** | ** | *** | 8 |
Sensitivity analysis
We used two sensitivity analyses (removal of one study and cumulative analysis) on the pooled analyses with a minimum of three studies included in the analysis. Both sensitivity analyses did not change the previous results and therefore showed the stability of the previous results.
Publication bias
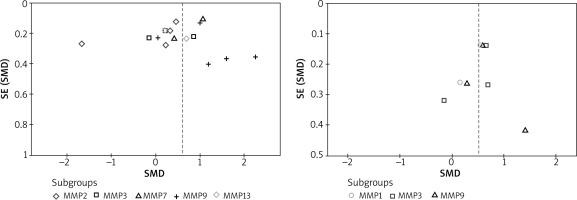
Both Egger’s and Begg’s tests were used for the analyses with a minimum of three studies included in the analysis. Both tests did not reveal any publication bias across the studies in all the analyses (Figure 4).
Discussion
The present meta-analysis evaluated the serum MMPs 2, 3, 7, 9, and 13 levels and the salivary MMPs 1, 3, and 9 levels. The results showed the serum MMPs 7 and 9 levels and also the salivary MMPs 1 and 9 levels were significantly higher in the OSCC patients than the controls. With regard to the salivary levels of MMPs in the OSCC patients compared to the controls, out of five studies [19, 21, 23, 24, 27] reporting the serum MMP2 level, two studies [19, 21] showed significantly elevated levels and one study [13] showed a significantly decreased level. Four studies [17, 18, 23, 27] reported the serum MMP3 level, from which two studies [18, 23] reported a significantly elevated level. The serum MMP7 level was reported in two studies [6, 27], of which one study [6] indicated a significantly elevated level. Out of five studies [19, 21, 24, 26, 27] reporting the serum MMP9 level, four studies [19, 21, 24, 26] showed significantly elevated levels. The serum MMP13 level was reported in two studies [23, 27], of which one study [13] showed a significantly elevated level. With regard to the salivary levels of MMPs in the OSCC patients compared to the controls, two [25, 26], three [4, 25, 26], and three studies [22, 25, 26] reported MMPs 1, 3, and 9 levels, respectively. The results showed that one [25], two [20, 25], and two studies [25, 26] showed significantly elevated levels.
One study [18] found no correlation between the serum MMP3 level and clinicopathological characteristics of the OSCC tumour, such as lymph node metastasis, size, grade, and stage; whereas, some previous studies have obtained a connection between MMP3 expression and clinicopathological characteristics of OSCC [28, 29] as well as levels of MMPs 1 and 10 [30]. Kurahara et al. [28] observed a direct correlation between MMP3 expression and incidence of lymph node metastasis. MMP13 is associated with the invasion depth and poor prognosis of OSCC [31]. A review study [32] showed that upregulation of MMPs 1 and 9 could be used as markers for malignant transformation to oral cancer. Studies have demonstrated that MMP13 is produced directly by the cancer tissue and indirectly advances the tumour angiogenesis [33, 34]. Baker et al. [35] proved that MMP3 expression was predominantly higher in OSCC than in normal tissue. A meta-analysis [36] on the Asian and European populations showed no significant connection between the MMP3 level and the risk of head and neck cancer, but in the European subgroup a MMP3 polymorphism was significantly related to the risk of head and neck cancer. Some reports have suggested that MMP9 expression may be an effective indicator for predicting the early stage of OSCC and also a good aim for therapeutic intervention [37, 38].
Vascular endothelial growth factor (VEGF) can increase MMP expression in endothelial cells [39]. A significant correlation has been found between serum levels of VEGF and MMP3 in the OSCC patients [18]. MMP9 may act as a pro-angiogenic indicator during tumorigenesis by raising the VEGF activity [40, 41]. Previous research has shown that tumour necrosis factor α and transforming growth factor β1 increase MMPs 2 and 9 RNA and protein expression levels in the lesion sites of OSCC [42, 43]. Zhang et al. [44] reported elevated levels of MMP1 in OSCC correlated fibroblasts compared with normal fibroblasts. Chemerin has been presented to stimulate MMP9 and other MMPs in human endothelial cells and chondrocytes, respectively [45, 46]. Thrailkill et al. [47] proposed a direct correlation between the serum MMP3 level and age. Also, one research [17] showed that the serum MMP3 level was higher in females than in males. Another research found different MMP3 serum levels in males and females [48]. These results show that age, sex, and other markers can affect MMPs levels and the pathological conditions of OSCC.
With regard to distinguishing OSCC from controls, the area under curve (AUC) rates obtained were 0.651 and 0.886 for serum MMPs 2 and 9, respectively [21]. Choudhry et al. [27] showed that the serum levels of MMPs 1, 2, 3, 7, 8, 9, 10, 12, and 13 had an AUC higher than 0.500. Another study [26] found an AUC of 1.00 for salivary and serum MMP9. Peisker et al. [22] reported an AUC of 0.698 for salivary MMP9. In addition, AUC was more than 0.500 for serum MMPs 2, 3, and 13 [23]. The results of AUC show that the MMP levels can distinguish OSCC from controls, which can be stronger or better than others based on some MMPs. Future studies are recommended to pay more attention to this point that MMPs can make higher differentiation.
Limitations: 1) Measurement of MMP levels was the second outcome in some studies. 2) Due to few studies reported, we could not conduct subgroup analysis. 3) Some studies had different treatments (chemotherapy, surgery, and radiotherapy) for patients and some studies had no treatment. 4) Different percentages were found for OSCC stages among the studies. 5) A number of studies included smokers and alcohol consumers as inclusion criteria. 6) There was high heterogeneity in most analyses. Strengths: 1) There was no publication bias. 2) The stability of the results was confirmed. 3) More than 60% of studies had high quality. 4) Most studies had a similar method for measurement of MMP levels. 5) In most studies, there were age- and/or sex-matched controls.
Conclusions
Considering the limitations and few studies reported, in particular, this meta-analysis showed that the serum MMPs 7 and 9 levels and the salivary MMPs 1 and 9 levels were significantly higher in the OSCC patients than in controls. However, due to high heterogeneity among the studies analysed in the meta-analysis, readers should construe the results more carefully and precisely. Future studies should be done with a larger sample size in different areas to confirm the usefulness of these MMPs in tumour detection or progression.