Purpose
Cervical uteri cancer is the second most common cancer among females in India. Sixty percent of cervical cancer patients present with locoregionally extensive clinical disease [1, 2]. Brachytherapy is an integral part of locally advanced cervical cancer management, following external beam radiotherapy (EBRT) and concurrent chemotherapy [3, 4]. Currently, 2-dimensional X-ray-based brachytherapy planning has largely been replaced by 3-dimensional image-guided brachytherapy [5]. Magnetic resonance imaging (MRI)-based adaptive radiotherapy is the gold standard of treatment in cervical intracavitary brachytherapy (ICBT) to reduce doses to organs at risk (OARs) to minimum, without compromising target coverage [6].
Several uncertainties prevail in image-guided brachytherapy (IGBT) planning, such as tumor regression between fractions, movement of OARs, and target in relation to applicator position and organ filling [7-9]. 3-dimensional computer tomography (CT)-based planning system, despite being available within several centers in developing nations, high patient load, and prevailing logistical reasons, prohibit its’ maximum utilization. Therefore, many centers are often compelled to use first IGBT plan in subsequent fractions and applications.
The current study addressed a major problem faced in developing countries due to large patient volume, lower resources, and technological challenges in patient care. Our study aimed to evaluate the feasibility and safety of executing optimized intra-cavitary brachytherapy (ICBT) plan of first fraction in subsequent fractions and applications.
Material and methods
Patient selection
After obtaining an approval from institutional review board, patients with biopsy-proven squamous cell carcinoma (SCC), staged according to FIGO (International Federation of Gynecology and Obstetrics) 2018 [10] were selected from November 2017 to September 2019. 30 patients with good response to EBRT, whose residual disease was confined to medial parametrium and cervix were included in the current study.
CT simulation and treatment overview
All patients received pelvic EBRT of 45-50 Gray (Gy) in 25 fractions by 3D-CRT (3-dimensional conformal radiotherapy) technique. Concurrent chemotherapy of cisplatin 40 mg/m2 was administered weekly for 5-6 cycles. One week post-EBRT, patients underwent ICBT application using Manchester/Fletcher-Suit high-dose-rate (HDR) remote afterloading applicator under spinal anesthesia. Vaginal packing was done to fix the applicator in position and to displace the bladder and rectum away. All patients underwent two such applications, one week apart.
Computed tomography simulation was performed using a 16-slice helical CT scanner. Axial scan of 2.5 mm slice thickness was obtained 1-2 hours before each treatment. Strict bladder and rectal protocols were followed, with 50 cc diluted iohexol contrast filled into the bladder via Foley’s catheter, and 20 cc contrast applied into the rectum.
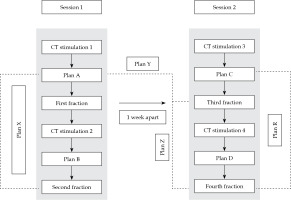
Two sets of plans were created for each application on images obtained at 18-24 hours gap (intra-application planning). Inter-applications plan sets were created for a second application performed after a gap of 7 days. Further details of the plans are presented in the following section and Figure 1.
Organs at risk were delineated on all acquired images. High-risk clinical target volume (HR-CTV) was based on MRI imaging findings at diagnosis, clinical examination under anesthesia (EUA) during brachytherapy (BT), and radiological findings from CT simulation scan, with the applicator in situ. A dose of 6.5 Gy was prescribed to point A at each fraction. This dose was further optimized to cover HR-CTV without compromising the dose to target volume, while respecting OARs’ tolerances (Figures 2-4). The accepted total dose limits as per our institutional protocol developed from the American Brachytherapy Society (ABS) and Indian Brachytherapy Society (IBS) guidelines, in equivalent 2 Gy dose (EQD2) [11, 12] were as follow:
Fig. 2
Coronal section of CT scan of ICBT application with isodose distribution. Represented isodose levels are 100% dose (orange), 90% (blue), and 80% (green)
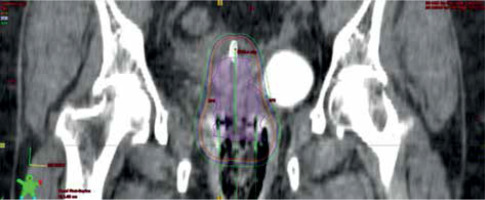
Fig. 3
Axial CT scan with applicator in place. Isodose levels of 650 cGy (orange) and 550 cGy (green) are presented
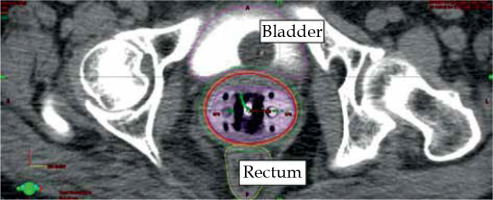
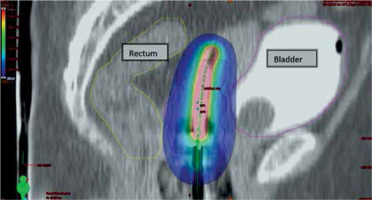
Fig. 4
Sagittal section of CT scan of ICBT application showing dose in color wash, blue shows prescribed dose 650 cGy
Dose to point A: EQD2 ≥ 75 Gy,
HR-CTV D90: EQD2 ≥ 80 Gy,
D2cc rectum: EQD2 ≤ 75 Gy,
D2cc bladder: EQD2 ≤ 85 Gy,
D2cc sigmoid: EQD2 ≤ 75 Gy.
Brachytherapy was delivered using iridium-192 with Gamma Med plus HDR remote afterloading device (Varian Medical System Palo Alto, CA, USA). ICBT treatment was performed in two sessions; in each session, one fraction was delivered on the first day of application, and second fraction was given on the subsequent morning, leaving the applicator in situ overnight, with a time gap of 18 to 24 hours between two fractions.
Comparative plans
The following plans were generated in CT simulation scans (Figure 1):
Plan A: An optimized plan in the first CT simulation.
Plan B: A new optimized plan in the second CT simulation was taken before the second fraction (single- application).
Plan C: A new plan optimized in the third CT simulation, taken before the third fraction (new application).
Plan D: A new plan optimized in the fourth CT simulation, taken before the fourth fraction (same application as in plan C).
Plan X: A plan with the same dwell positions and timings (corrected for source decay) as the first fraction (plan A), and applied to the second CT simulation.
Plan Y: A plan generated with the same dwell positions and timings (corrected for source decay) as the first fraction (plan A), and applied to the third CT simulation.
Plan Z: A plan generated with the same dwell positions and timings (corrected for source decay) as the first fraction (plan A), and applied to the fourth CT simulation.
Plan R: A plan generated with the same dwell positions and timings (corrected for source decay) as of the third fraction (plan C), and applied to the fourth CT simulation.
Intra-application variation: Dose variation between plan X and plan B; plan R and plan D; plan A and plan X.
Inter-application dose variation: Dose variation between plan Y and plan C; Dose variation between plan Z and plan D.
Dosimetric parameters were recorded for each fraction in point A doses, D2cc bladder, rectum, and sigmoid.
Statistical method
Normality of data sets was analyzed and confirmed using Shapiro-Wilk test. Dosimetric parameters were expressed in terms of mean and standard deviation. Inter-application dosimetric variation and intra-application dosimetric variation were analyzed with paired t-test for comparison of means. Statistical tests were performed using Statistical Package for the Social Sciences (SPSS) 21 software. Any p-value of less than 0.05 was considered statistically significant.
Results
Patient characteristics at baseline are elaborated in Table 1. Among 30 patients, ten patients underwent all the planned four simulations, and the remaining 20 patients underwent three CT simulations (first three), owing to unavailability of a dedicated CT simulator in our department. Therefore, the plans were compared as described in Figure 1 for ten patients, with all four image sets. For the remaining 20 patients, intra-application variation was evaluated between the first and second fractions. Unfortunately, a fourth scan could not be obtained due to logistic limitations. Hence, plan Z and plan R could not be generated for comparison.
Table 1
Patients’ profile (n = 30)
| Age (years) | Range | 32-55 |
| Median | 46 | |
| Stage (number of patients) | I | 0 |
| IIA | 3 | |
| IIB | 23 | |
| IIIA | 0 | |
| IIIB | 4 | |
| Histologic type (number of patients) | Squamous cell carcinoma | 30 |
Intra-application variation: In 40 sets of intra-application plans, 2 cc sigmoid dose was found to be significantly higher, if the first plan was executed on the second day without imaging and optimization. There was a dose reduction and mean difference of 50 ±114 cGy, with a p-value of 0.021 (Table 2). When a single plan was executed on consecutive days, doses to 2 cc sigmoid presented a wide range of variation compared to optimized plans, where variation was minimal (Figure 5). Also, there was a significant reduction in dose to 2 cc bladder in plan A compared to plan X, with a p-value of 0.007 with a mean 2 cc doses of optimized and unoptimized plans being 511 ±85.5 cGy and 553.6 ±111.2 cGy, respectively (Table 3).
Fig. 5
Box-and-Whisker plot representation of intra-application variation plan X and plan B in sigmoid dose (SI = sigmoid)
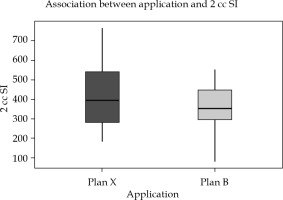
Table 2
Intra-application variation between plan X and plan B
Table 3
Intra-application variation between plan A and plan X
Inter-application variation: Doses to OARs, particularly 2 cc rectum and 2 cc sigmoid, showed significant reduction in the dose in new optimized plans. Mean difference of 2 cc rectal dose was 51.39 cGy (±85), with a p-value of 0.002, and 2 cc sigmoid was 46.9 cGy (±90), with p-value of 0.007, both p-values being statistically significant (Figures 6 and 7, Table 4).
Fig. 6
Box-and-Whisker representation of inter-application dose variation of rectum plan Y and plan C (R = rec- tum)
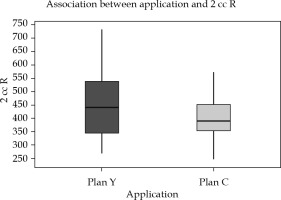
Fig. 7
Box-and-Whisker representation of inter-application dose variation of the sigmoid in plan Y and plan C (SI = sigmoid)
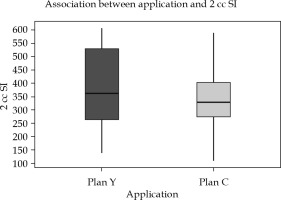
Table 4
Inter-application variation between plan C and plan Y
All possible combinations of executed and applied plans for the 40 sets of scans in 10 patients were compared. The total mean EQD2 values were obtained as follows: 79 Gy for left point A (when single-plan dwell time and position applied in further scans), and 78 Gy for left point A (with imaging and optimization for each fraction). Similarly, the mean EQD2 of right point A with a single-plan was 78.5 Gy, and with an individualized plan was 77.5 Gy. Hence, point A doses were more or less similar between optimized and non-optimized plans, but without statistical significance.
EQD2 of the bladder, rectum, and sigmoid exceeded tolerance limit in 4, 3, and 3 patients, respectively. 7 out of 30 patients had OARs’ doses exceeding tolerance limits. Two patients presented with more than one organ receiving higher doses.
In Figures 5-7, Box-and-Whisker plot depicts the distribution of 2 cc OAR doses between two plans. The middle horizontal line represents the median 2 cc OARs’ doses, and the upper and lower bounds indicate the 75th and the 25th centile, respectively.
Discussion
MRI-based image-guided adaptive planning for each fraction is the existing optimal approach for cervical cancer HDR-BT treatment [11]. According to Pötter et al. [5], adaptive MRI planning has provided local control rates of 95-100% at three years, with ICBT in limited-stage disease (IB/IIB); 85-90% with combined intra-cavitary + interstitial brachytherapy in advanced stages or poor response cervix cancer patients, with minimal treatment-related morbidity. However, CT scan is commonly used for target and OARs delineation due to its’ wide availability and accessibility. Therefore, in the current study, CT-based contouring has been followed using the available guidelines [13, 14].
Grigsby et al. [15] observed a significant anatomical variation in gynecologic brachytherapy prescription points, because of changes in uterine axis, slippage of tandem and colpostat, patient movements, vaginal packing dislodgment, tumor regression between multiple fractions, and gaseous distention of the bowel. With all these uncertainties, our study attempted to investigate the feasibility and safety of the execution of previous day plan in subsequent fraction without any change in target coverage and OARs toxicity.
Al-Booz et al. [16] noted an increase in dose to the sigmoid in ICBT, when point A doses were not compromised. Similarly, the sigmoid colon was consistently observed to receive unknown variable doses in the current study if subsequent fractions were not imaged and planned. Chakraborty et al. [17] fused two scan images and structure sets, and compared their plans with multiplanar reconstruction. 16% of their patients had their OARs tolerances exceeded, as the second fraction was not imaged. In our study, EQD2 of OARs exceeded in 23% of the patients (multiple patients had more than one organ crossing tolerance limits). Single-plan execution in subsequent applications by Kirisits et al. resulted in an average increase of D2cc doses to bladder by 3.5 Gy, rectum by 2.8 Gy, and sigmoid by 5.8 Gy [18]. Additionally, a relative increase in D2 cc to the bladder by 9% was observed when a uniform bladder filling protocol was not followed [18]. Therefore, a constant bladder filling protocol was adopted in our study. However, variation in doses would have resulted from other uncertainties, such as alteration in volume of the vaginal packing due to vaginal or uterine secretions, and differential patient’s hydration.
Mohammed et al. [19] evaluated the feasibility of applying a dose-optimized plan of ICBT and ISBT (interstitial brachytherapy) PDR (pulsed-dose-rate) brachytherapy of a first fraction to subsequent fraction. They intended to limit the number of scans and plans generated. Among 32 ICBT patients, dose delivered to 100% of target (D100) and volume of target receiving 100% of prescription dose (V100) were significantly different. OARs doses were outside the tolerance limit for 5 out of 32 patients with a single-plan. Moreover, Chi et al. demonstrated a statistically significant increase in the absolute D2cc dose to the bladder by 4.6 Gy and the rectum by 6.4 Gy, when a single-ICBT plan was executed in subsequent fractions [20]. In the current study, if a single-plan was applied for subsequent fractions, 10% of patients (3/30) would receive EQD2 to 2 cc sigmoid and 2 cc rectum beyond the level of tolerance. Even though it was not statistically significant, the total bladder dose in 3 of 30 (10%) patients showed an increase of 2-8 Gy EQD2 dose.
Centers with limited imaging access to brachytherapy may assume that it is safe to carry on brachytherapy insertions and treatment without imaging and re-planning for each insertion. However, the current study emphasizes that such a practice would be unsafe due to an increase in OARs doses. This, in turn, may contribute to several acute and late radiation toxicities. Therefore, it is suggested that centers with logistic challenges should explore other possibilities, including prior coordination with a radiology department or obtaining dedicated time slots on imaging machines for ICBT cases.
Drawbacks of the current study include a small sample size and the inability to acquire the fourth CT scan for 20 patients, owing to the lack of a dedicated CT simulator in our department. MRI at BT with applicator in situ, or even before BT, could not be obtained due to availability of a single-MRI unit designated for the entire hospital, and non-availability of MR-compatible applicators. Applicator geometry, organ deformations, and target response are few confounding factors, which could affect dose distributions. Further studies are required to analyze the impact of these factors upon inter-application and intra-application dose variations.
Dedicated CT simulators and MR-compatible applicators have recently been acquired at our Institute. Based on the outcomes of the current study, we have adopted brachytherapy planning prior to each fraction.