Introduction
Stomach cancer, medically referred to as gastric cancer, is distinguished by the excessive growth of cells that originate in the gastric region [1]. There exist specific genes that may harbor hereditary mutations, thereby increasing an individual’s vulnerability to developing stomach cancer. Nevertheless, the majority of genetic modifications linked to gastric cancer occur after birth due to risk factors such as infection with Helicobacter pylori or the consumption of tobacco [2, 3]. On a global scale, the yearly occurrence of gastric cancer exceeds one million instances. Stomach cancer is globally recognized as the fifth most commonly diagnosed cancer, and it is ranked seventh in terms of prevalence [4]. Based on a comprehensive global study, it has been determined that the cumulative probability of males developing gastric cancer over their lifetime is 1.87%, whereas for females, the corresponding risk is 0.79% [5].
During the early phases of cancer progression, surgical intervention is frequently utilized as a therapeutic approach. This commonly entails the execution of either subtotal gastrectomy, which involves the excision of a segment of the stomach, or total gastrectomy, which encompasses the complete removal of the stomach in addition to the nearby lymph nodes [6, 7]. At present, a diverse range of interventional strategies are being employed during the perioperative phase of gastric cancer, with the aim of promoting patients’ recuperation.
The pioneering of the concept of enhanced recovery after surgery (ERAS), also known as fast track surgery (FTS), can be attributed to Henrik Kehlet in the 1990s [8]. In recent times, there has been a notable progression in the methodology owing to its significant benefits and elevated degree of security [9]. The ERAS protocol is a comprehensive and interdisciplinary approach aimed at reducing the physiological stress response and organ dysfunction that often accompany surgical procedures. Its primary objective is to promote the recovery of patients following surgery [10]. The core components of ERAS encompass a range of factors, including anesthesia and perioperative fluid management, efficient pain control, prompt initiation of oral food intake, and early mobilization, among other considerations [11]. Recently, there has been an expansion in the application of ERAS protocols across different surgical fields, such as radical prostatectomy, cardiac surgery, and colorectal surgery [12, 13]. Previous studies have demonstrated that the implementation of ERAS protocols holds promise in facilitating the postoperative recovery period for patients undergoing both open and laparoscopic-assisted gastrectomy (LAG) procedures for gastric cancer [14, 15].
However, the precise influence of ERAS on LAG remains unclear.
Aim
This meta-analysis aims to assess the viability and safety of ERAS in patients with gastric cancer who are undergoing gastrectomy, in comparison to conventional care. The study selects randomized controlled trials (RCTs) [16–26] based on specific inclusion and exclusion criteria.
Material and methods
The current study adhered to the guidelines set forth by PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [27].
Search strategy
A comprehensive and methodical examination of randomized controlled trials (RCTs) was undertaken on the databases of PubMed and the Cochrane Library, adhering to the guidelines stipulated in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The search was conducted using the specified keywords: “Enhanced recovery after surgery” or “ERAS”; “Fast track surgery” or “FTS”; “Gastric cancer surgery” or “gastrectomy”; “meta-analysis”; “post-operative hospital stay”; “post-operative complications”; “readmission rate”; “mortality rate”. The researchers performed an extensive examination of scholarly literature by employing the databases of PubMed and Cochrane Library. The integration of the Medical Subject Headings (MeSH) and textual keywords was accomplished by employing the Boolean operator “AND” within the context of the search strategy. The utilization of Boolean operators, namely AND, OR, and NOT, facilitated the amalgamation of words and phrases in order to restrict, expand, or delineate the scope of the search.
Additionally, two researchers (YW and SL) conducted a thorough bibliographic search to identify pertinent and influential scholarly articles. A rigorous methodology was employed to identify and include all randomized controlled trials (RCTs) published from 2010 to 2023, while also aggregating relevant findings from primary qualitative studies.
Inclusion and exclusion criteria
The present study encompassed a comprehensive review of pertinent scholarly works published from 2012 to 2023, focusing on the comparative outcomes of individuals diagnosed with gastric cancer who underwent gastrectomy utilizing the ERAS protocol in contrast to those who received conventional care. The researchers placed emphasis on the incorporation of complete textual content articles within their study. The inclusion of abstracts in the meta-analysis was contingent upon the provision of adequate information. The analysis excluded studies that had insufficient data, lacked relevance to gastric cancer surgery, or were published prior to 2010. The authors YW and SL performed a comprehensive literature review to identify relevant studies. The researchers utilized inclusion criteria to exclude references that were outdated and to incorporate studies that were of significant importance.
Evaluation of the analytical variables
The researchers YW and SL independently collected the demographic summary and event data from the included studies. The main results were as follows: the variables of interest in this study include: (1) the overall length of hospitalization following surgery; (2) the cumulative count of complications that arise after surgery; (3) the time until the first occurrence of flatus; (4) the time until the first oral intake of food; (5) the time until the first instance of ambulation; (6) the rate of patients being readmitted to the hospital; and (7) the rate of mortality.
Sources of heterogeneity
Two reviewers, YW and SL, independently evaluated the methodological validity of the studies included in the analysis. The author, SW, successfully resolved any disagreements that arose between YW and SL. The calculation of heterogeneity was performed among the experiments that were included. The examination of heterogeneity was conducted by employing the Cochran Q statistic and I2 index in a random bivariate mode [28], with the aid of the RevMan software [29]. Multiple sources of heterogeneity were investigated, encompassing the comparison between full-text publications and abstracts, disparities in age groups and sample sizes, discrepancies in the evaluated surgical parameters, and variations in study outcomes.
Risk of bias assessment
The assessment of potential bias in the studies included in the analysis was conducted using a standardized questionnaire that had been previously established. The researchers utilized the Cochrane Risk of Bias: Robvis Tool [30] to produce a concise summary and graphical representation of the risk of bias.
Meta-analysis
The meta-analysis was performed utilizing the RevMan software (Review Manager, RevMan, Version 5, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2020). The group exhibiting a degree of heterogeneity exceeding 50% made the decision to employ the random effect, while the subgroup with heterogeneity below 50% opted for the fixed effect. The main approach utilized in this study involved the implementation of the Mantel-Haenszel technique, incorporating random bivariate effects. The aforementioned method was primarily utilized to calculate statistical measures such as standard deviation and odds ratio, along with a 95% CI [31]. In addition, forest plots were generated to visually depict the aforementioned findings. The metrics used by the researchers to evaluate the extent of heterogeneity in the analyzed studies included τ2, χ2, I2, and z values. Statistical significance was determined by considering a p-value below the predetermined threshold of 0.05. The DerSimonian and Laird method was utilized to compute the diagnostic odds ratio using a 2 x 2 contingency table [32]. The evaluation of publication bias in the studies that were included in the analysis was performed through the utilization of Begg’s test [33], Egger’s test [34], and Deek’s funnel plot [35]. The Deek’s funnel plot was constructed by plotting the logarithm of the odds ratio for each individual study against its corresponding standard error, utilizing MedCalc software [36].
Results
Literature search results
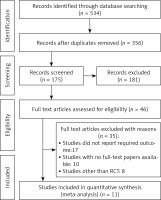
The PRISMA flowchart, as depicted in Figure 1, is utilized for the purpose of selecting research studies in the research process. Following an extensive examination of online databases, a total of 534 studies were identified. After eliminating duplicate entries, a comprehensive set of 353 studies was selected for screening based on their abstracts and titles. A thorough assessment was conducted on a total of 142 studies that satisfied the predetermined criteria for inclusion. The present meta-analysis consisted of a total of 11 studies, which were chosen according to predetermined criteria for inclusion and exclusion. The studies included in this analysis evaluated the efficacy and safety of the ERAS protocol in patients who underwent gastrectomy for gastric cancer. The analysis exclusively incorporated randomized controlled trials as the primary source of the included studies. Table I provides a comprehensive summary of the relevant attributes of the studies under investigation. The attributes encompass the identification of the studies, including their publication years, study designs, journals of publication, total participant count, participant distribution between the ERAS and control groups, age distribution within both groups, gender ratio, type of surgery performed, and primary outcome measures.
Table I
Characteristics of the included studies
| Study ID | Year of publication | Type of study | Journal of publication | Total participants | Age of participants | Gender M/F | Participants in EG | Participants in CG | Type of surgery | Primary outcomes# | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| EG | CG | ||||||||||
| Abdikarim et al. [16] | 2015 | RCT | World Journal of Gastroenterology | 61 | 63 ±12 | 62 ±11 | 41/20 | 30 | 31 | Laparoscopic radical gastrectomy | 1, 2,4,5,6,7 |
| Cao et al. [17] | 2021 | RCT | Journal of Surgical Research | 171 | 70.8 ±3.4 | 71.4 ±3.7 | 109/62 | 85 | 86 | Laparoscopic total gastrectomy | 1,2,3,4,5,6 |
| Desiderio et al. [18] | 2018 | RCT | Journal of Gastric Cancer | 60 | 61 ±16 | 63 ±14 | 27/33 | 20 | 40 | Laparoscopic distal gastrectomy | 1,2,3,4,5,6,7 |
| Giovanardi et al. [19] | 2020 | RCT | Journal of Gastric Surgery | 440 | 67.14 ±13.03 | 67.18 ±12.62 | 260/180 | 220 | 220 | Laparoscopic distal gastrectomy | 1,2,3,4,5,6 |
| Kang et al. [20] | 2018 | RCT | Annals of Surgical Oncology | 97 | 56.3 ±10.4 | 54.5 ±12.6 | 71/26 | 51 | 46 | Laparoscopic distal gastrectomy | 1,2,3,4,5,6 |
| Kim et al. [21] | 2012 | RCT | World Journal of Surgery | 47 | 57.45 ±14.54 | 52.64 ±11.57 | 28/16 | 22 | 22 | Laparoscopic distal gastrectomy | 1,2,3,4,5 |
| Liu et al. [22] | 2016 | RCT | OncoTargets and Therapy | 84 | 70.3 ±5.8 | 69.2 ±5.1 | 42/42 | 42 | 42 | Laparoscopic radical gastrectomy | 1,2,3 |
| Tian et al. [23] | 2022 | RCT | Annals of Surgery | 400 | 58.3 ±10.5 | 58.6 ±10.9 | 253/117 | 200 | 200 | Laparoscopic distal gastrectomy | 1,2,3,4,5,6,7 |
| Wang et al. [24] | 2019 | RCT | Brazilian Journal of Medical and Biological Research | 60 | 58.22 ±4.31 | 59.26 ±5.35 | 48/12 | 30 | 30 | Laparoscopic radical gastrectomy | 1,2,3,4 |
| Yamada et al. [25] | 2012 | RCT | Gastric Cancer | 191 | 65 ±3.97 | 59.26 ±3.14 | 134/57 | 91 | 100 | Laparoscopic radical gastrectomy | 1,2,3,4, 6 |
| Yoshikawa et al. [26] | 2023 | RCT | World Journal of Surgical Oncology | 182 | 67.3 ±1.08 | 67.4 ±1.36 | 136/46 | 111 | 71 | Robotic and laparoscopic distal gastrectomy | 1,2,3,4,5,6 |
Quality assessment of the included studies
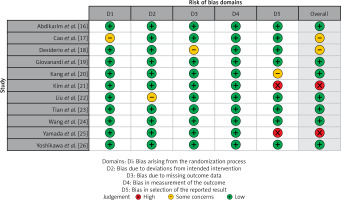
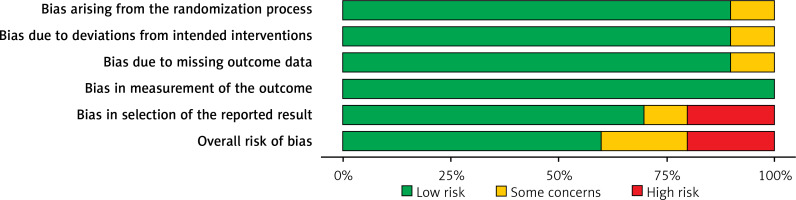
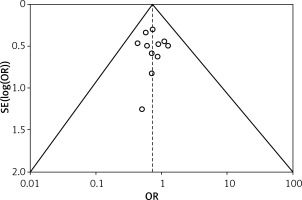
Table II displays the evaluation of the quality of the studies that were incorporated in this meta-analysis. Figure 2 provides a concise overview of the risk of bias, while Figure 3 presents a visual depiction of the risk of bias. Out of the total of eleven studies that were included in the analysis, it was found that seven of them demonstrated a low susceptibility to bias. However, it has been noted that two studies demonstrated a moderate risk of bias due to concerns regarding the randomization process and the absence of outcome data. The two remaining studies were found to have a significant risk of bias in terms of the selection of the outcomes reported. The results presented in Figure 4 suggest that there is limited evidence of publication bias, as demonstrated by the lack of statistical significance (p > 0.05) in both Begg’s test (p = 0.424) and Egger’s test (p = 0.217) [37].
Table II
Assessment of bias for included studies
| Variable | Abdikarim et al. [16] | Cao et al. [17] | Desiderio et al. [18] | Giovanardi et al. [19] | Kang et al. [20] | Kim et al. [21] | Liu et al. [22] | Tian et al. [23] | Wang et al. [24] | Yamada et al. [25] | Yoshikawa et al. [26] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Did the study avoid inappropriate exclusions? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Did all patients receive the same reference standard? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Were all patients included in the analysis? | N | N | N | N | N | N | N | N | N | N | N |
| Was the sampling frame appropriate to address the target population? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Were study participants sampled in an appropriate way? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Were the study subjects and the setting described in detail? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Were valid methods used for the identification of the condition? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Was the condition measured in a standard, reliable way for all participants? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
Statistical analysis of the primary outcomes
The current meta-analysis comprised a sample of eleven randomized controlled trials, encompassing a total of 1790 participants. Among the entire sample, ERAS care was administered to 902 individuals, while conventional care was provided to 888 individuals. Table III succinctly presents the ERAS components that were integrated into the selected RCTs. The statistical analysis was conducted on the primary outcomes of the studies included in order to evaluate the safety and effectiveness of ERAS for gastrectomy in patients diagnosed with gastric cancer.
Table III
ERAS elements applied in the included studies
| Variable | Abdikarim et al. [16] | Cao et al. [17] | Desiderio et al. [18] | Giovanardi et al. [19] | Kang et al. [20] | Kim et al. [21] | Liu et al. [22] | Tian et al. [23] | Wang et al. [24] | Yamada et al. [25] | Yoshikawa et al. [26] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-admission information and counselling | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |
| Pre-operative bowel preparation | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Pre-operative fasting and pre-operative carbohydrate loading | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Preanesthetic medication | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Prophylaxis against thromboembolism | √ | √ | √ | √ | √ | √ | √ | ||||
| Antimicrobial prophylaxis | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Standard anesthetic protocol | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Preventing and treating post-operative nausea and vomiting | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Laparoscopy-assisted surgery | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Surgical incision | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Nasogastric intubation | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Preventing intraoperative hypothermia | √ | √ | √ | √ | √ | ||||||
| Peri-operative fluid management | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Drainage of peritoneal cavity following colonic anastomosis | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Urinary drainage | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Prevention of post-operative ileus | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Post-operative analgesia | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Post-operative nutritional care | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Early mobilization | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Audit | √ | √ | √ | √ | √ | √ | √ |
Length of post-operative hospital stay
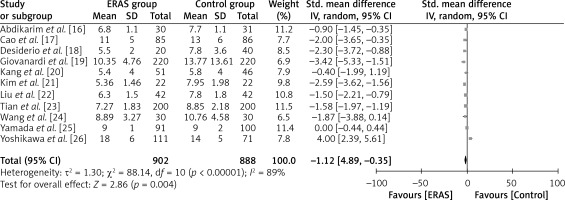
Figure 5 illustrates the inclusion of eleven studies that have reported the aforementioned outcome. The ERAS group consisted of a total of 902 patients, while the standard treatment group comprised 888 patients. The results of the study indicate that patients who underwent ERAS had a significantly shorter duration of post-operative hospitalization compared to patients who received standard care. This conclusion is supported by WMD of –1.12 days, with a 95% CI ranging from –1.89 to –0.35 days. The p-value, which represents the statistical significance, was found to be less than 0.00001. The analysis employed a random-effects model to account for high heterogeneity, as indicated by an I2 value of 89%.
Total number of post-operative complications
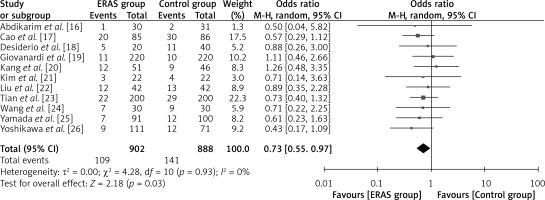
Eleven studies reported this result, with a total of 902 patients in the ERAS group and 888 patients in the conventional care group, as depicted in Figure 6. As indicated by the odds ratio (OR) of 0.73 (95% CI: 0.55 to 0.97; p = 0.03), the number of ERAS patients who experienced post-operative complications was lower than that of patients receiving conventional care. A random-effects model was utilized with I2 = 60%, indicating substantial heterogeneity.
Time to first flatus
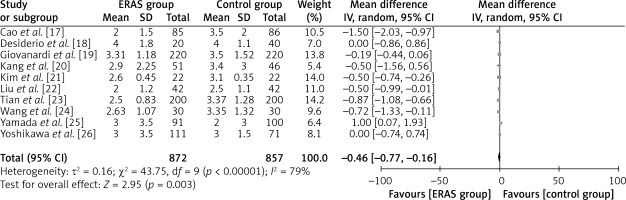
The aforementioned parameter was documented in ten separate studies, encompassing a collective sample size of 872 patients in the ERAS group and 857 patients in the traditional care group (Figure 7). The findings of the study indicated that patients who underwent ERAS demonstrated a faster recovery in terms of resumption of flatus production compared to patients who received conventional care. The evidence presented in this study supports the conclusion that there is a statistically significant difference in mean values, as indicated by WMD of –0.46 days, a 95% CI ranging from –0.77 to –0.16 days, and a p-value less than 0.00001. A random-effects model was employed due to the significant heterogeneity observed (I2 = 79%).
Time to first oral food intake
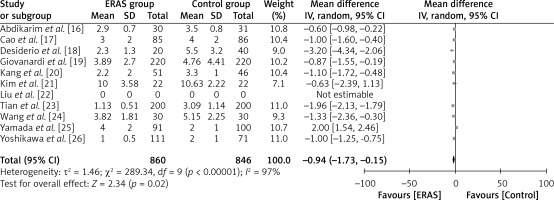
A total of 860 patients in the ERAS group and 846 patients in the conventional care group were included in the analysis across ten studies, as illustrated in Figure 8. The findings of this study indicate that patients who undergo ERAS consume food within a shorter time compared to patients who receive conventional care. This conclusion is supported by a statistically significant WMD of –0.94 days (95% CI: –1.73 to –0.15; p = 0.02). The study employed a random-effects model to account for significant heterogeneity, as indicated by an I2 value of 97%.
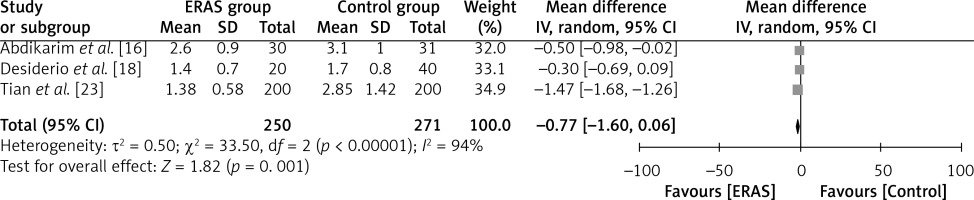
Time to ambulation
The results of three investigations are illustrated in Figure 9, where a total of 250 patients were assigned to the ERAS group and 271 patients were assigned to the conventional care group. According to the results of the study, the WMD between ERAS patients and traditional care patients was –0.77 days (95% CI: –1.60 to –0.06, p = 0.001). This indicates that ERAS patients had a shorter duration of ambulation compared to patients receiving traditional care. The researchers employed a random-effects model in their analysis, and the value of I2 was found to be 94%, indicating a significant level of heterogeneity.
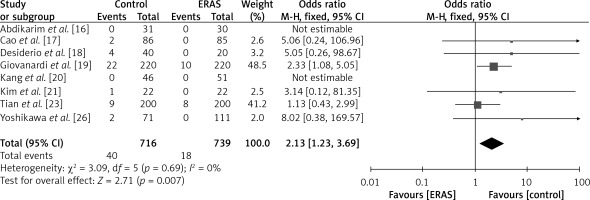
Readmission rate
The findings of eight investigations, encompassing a collective sample size of 716 patients in the ERAS group and 739 patients in the conventional care group, are depicted in Figure 10. The fixed-effects model was employed due to the reduced heterogeneity (I2 = 0%). Based on the OR of 2.13 (95% CI: 1.23 to 3.69, p = 0.005), it can be inferred that patients in the control group had a comparatively lower rate of readmission compared to those in the ERAS group.
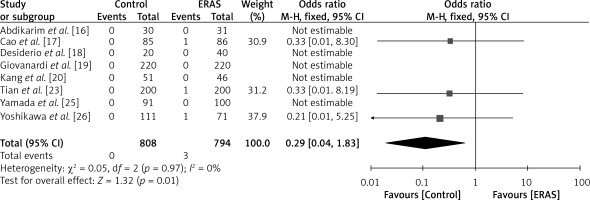
Mortality rate
A total of eight studies included in the analysis reported the aforementioned outcome, encompassing a cohort of 808 patients in the ERAS group and 794 patients in the traditional care group, as depicted in Figure 11. In this study, a fixed-effects model was utilized, and the heterogeneity value (I2) was found to be low at 0%. The findings indicated that the mortality rate was lower among patients in the control group compared to those in the ERAS group, as demonstrated by an OR of 0.29 (95% CI: 0.04 to 1.83, p = 0.01).
Discussion
The concept of enhanced recovery after surgery (ERAS) was initially proposed by the Danish physician Kehlet [37]. The primary objective of ERAS is to minimize surgical trauma and promote efficient postoperative recuperation through the implementation of a comprehensive array of perioperative strategies. The essential components of ERAS encompass various aspects such as educating patients and their families, optimizing patients’ condition before admission, implementing a reduced fasting period that involves consuming a carbohydrate beverage 2 h prior to anesthesia, utilizing multimodal analgesia with judicious use of opioids when necessary, facilitating a prompt return to regular diet and activities on the day of the surgery, and enabling patients to be discharged and return home [38, 39]. In contemporary times, laparoscopic surgery has attracted significant interest as compared to open surgery due to its superior short-term results. The aforementioned advantages include a decrease in surgical bleeding, accelerated recovery of bowel function, relief of pain, reduced occurrence of complications, and shorter hospital stays after the procedure [40–42]. Currently, the ERAS and laparoscopic techniques has been extensively employed. Nevertheless, the efficacy of ERAS in individuals undergoing laparoscopic gastrectomy remains uncertain.
In contrast to prior meta-analytical inquiries that have examined both open and laparoscopic gastrectomy, the current study focuses solely on the surgical approach of laparoscopic-assisted gastrectomy. As an example, Ding et al. [43] conducted a comprehensive review and meta-analysis, encompassing a sample of eight studies and a total of 801 patients. The researchers discovered that the ERAS group exhibited a statistically significant reduction in the time to first flatus (WMD = –14.57; 95% CI: –20.31 to –8.83, p = 0.00001), a decrease in the duration of postoperative hospital stays (WMD = –1.85; 95% CI: –2.35 to –1.35, p = 0.00001), and a decrease in hospital charges (WMD = –0.94, 95% CI: –1.40 to 0.49, p = 0.0001). Nonetheless, the researchers also noted a higher frequency of readmissions in the ERAS group, exhibiting an odds ratio of 3.42 (95% CI: 1.43 to 8.21, p = 0.006). The researchers have documented the ERAS protocol as a secure and efficacious approach for performing gastrectomy in patients diagnosed with gastric cancer. Nevertheless, it has been recommended that in order to derive reliable and sound conclusions, additional investigations should be undertaken using multicenter and randomized trials.
In a systematic review and meta-analysis conducted by Li et al. [44], it was observed that patients who underwent LAG and adhered to an ERAS protocol exhibited a decrease in the duration of postoperative hospitalization (WMD = –2.16; 95% CI: –3.05 to –1.26, p < 0.00001) as well as lower hospitalization costs (WMD = –4.72; 95% CI = –6.88 to –2.55, p = 0.00001). The findings of the research demonstrate that the intervention resulted in a statistically significant reduction in postoperative complications (p < 0.00001), a decreased duration until the first occurrence of flatus (WMD = –9.72; 95% CI: –13.75 to –5.81, p < 0.00001), an expedited overall recovery without an associated rise in complications or readmission rates, and an enhanced pace of clinical recovery among individuals diagnosed with gastric cancer. According to Yamagata et al. [45], the adoption of the ERAS protocol was found to be associated with reduced hospital expenses and length of stay, without compromising the incidence of surgical complications. The aforementioned discovery implies that the ERAS protocol exhibits potential as a viable approach for surgical treatment of gastric cancer. In their study, Pędziwiatr et al. [46] found that laparoscopic gastrectomy, when combined with the ERAS protocol, can be a beneficial alternative to the traditional approach. The authors observed this in a sample of eleven patients who underwent laparoscopic D2 gastrectomy, noting a reduction in hospital stay as a result. In a study conducted by Pisarska et al. [47], it was found that the utilization of laparoscopy in conjunction with the ERAS protocol in patients with gastric cancer who underwent elective laparoscopic total gastrectomy resulted in favorable clinical outcomes.
The decision to utilize LAG was informed by a body of empirical research [48–50], which consistently demonstrates a significant correlation between LAG and a reduced incidence of postoperative complications, including incision infection and pneumonia, when compared to open gastrectomy (OG). The findings of the meta-analysis indicate that the ERAS group exhibits a statistically significant decrease in the length of postoperative hospital stay, a reduced incidence of postoperative complications, a shorter time to first passage of gas, a shorter time to first oral food intake, and a shorter time to first ambulation compared to the conventional care group. Furthermore, the group of patients who underwent the ERAS exhibited lower rates of readmission and mortality when compared to the group of patients who received standard care. The assessment of the safety and feasibility of a surgical procedure primarily depends on the occurrence of postoperative complications and the prompt initiation of normal metabolic processes [51, 52]. ERAS models encompass multi-modal perioperative care pathways. The primary objective of their design is to facilitate prompt recuperation subsequent to surgical interventions through the preservation of preoperative organ functionality and the mitigation of the substantial physiological stress reaction that ensues post-surgery. ERAS encompasses a set of strategies that prioritize patient engagement, preoperative medical optimization, optimal anesthesia protocols, and prophylaxis against postoperative nausea. In a study conducted by Lohsiriwat et al. [53], the implementation of the ERAS protocol resulted in a shorter median hospital stay for patients following surgery. The median hospital stay for patients in the ERAS group was 5.5 days, with a range of 3 to 16 days, compared to 7.5 days, with a range of 5 to 25 days, for patients not in the ERAS group.
However, in a study by Gumusoglu et al. [54] suggested that interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), C-reactive protein (CRP), and procalcitonin (PCT) have demonstrated potential as biomarkers for the timely identification of significant complications in gastric cancer patients who are undergoing the ERAS protocol. Imaging modalities ought to be employed in individuals exhibiting elevated concentrations of these inflammatory biomarkers on the third and fifth postoperative day.
Based on the notable reduction in postoperative complications observed among patients in the ERAS group, it is suggested that the ERAS protocol be considered as a prospective approach for implementation during gastrectomy procedures in individuals diagnosed with gastric cancer.
The present meta-analysis is constrained by various limitations. The findings of this study are limited due to the inclusion of a small number of randomized controlled trials, specifically 11, which exhibit moderate to high levels of heterogeneity, despite the researchers’ commitment to adhering to recommended methodological rigor. The effectiveness of the ERAS program is contingent upon the proficiency and cooperation of a diverse group of healthcare professionals, including anesthesiologists, surgeons, dieticians, and skilled nursing personnel. Given the inclusion of numerous clinical centers and surgeons, this study presents the potential for variations in the execution and results of the ERAS program. As a result of the existence of heterogeneous populations and disparate reported outcomes, it is plausible for there to be differences in pre- and post-operative variables. Furthermore, the scope of our meta-analysis may have been constrained by the inclusion of solely articles written in the English language. Finally, it is important to acknowledge that the applicability of the results to a broader population is constrained due to the limited number of studies and patient cohorts encompassed in this analysis. Hence, further investigation is warranted to delve deeper into this matter.
Conclusions
The findings of this meta-analysis indicate that the utilization of Enhanced Recovery After Surgery in combination with either robotic or laparoscopic surgical techniques is a viable and efficacious option for managing gastric cancer. Moreover, it possesses the capacity to significantly diminish diverse postoperative variables including the length of post-operative hospitalization, occurrence of post-operative complications, time until the first occurrence of flatus, time until the first oral food intake, time until ambulation, rates of readmission, and mortality rates in comparison to traditional care approaches. The findings of our study suggest that the ERAS protocol exhibits effectiveness and safety in promoting the recovery of typical physiological processes in individuals who have undergone gastrectomy for gastric cancer. Nevertheless, it is crucial to undertake rigorous and extensive research endeavors in order to acquire more substantial and reliable evidence.