Introduction
Syndromic immunodeficiencies may present with varying clinical manifestations indicative of immune failure, as well as distinct clinical signs of multiple-organ abnormalities. These immunodeficiencies may occur concomitantly with various conditions, such as metabolic/teratogenic disorders or chromosomal abnormalities. Affected patients usually exhibit recurrent sinopulmonary infections, and immunodeficiency is detected after diagnosis of the underlying syndrome. However, immunodeficiency may not be present in all syndromic patients [1]. The recognition of a syndrome that affects both immune and organ systems can facilitate accurate diagnosis and treatment. Well-defined syndromic immunodeficiencies (e.g., ataxia- telangiectasia and Wiskott-Aldrich syndrome), which are accompanied by organ dysfunction and dysmorphic syndromes, constitute a subgroup of immunodeficiencies [2, 3]. Syndromic immunodeficiencies may involve T-cell and B-cell deficiencies, as well as phagocytic disorders, complement deficiencies, or innate immune defects.1 To our knowledge, there is minimal information regarding the immunological abnormalities in rare syndromes which have not been classified as “well-defined syndrome with immunodeficiency” by the IUIS Phenotypical Classification for Human Inborn Errors of Immunity yet. This study aimed to evaluate immunological abnormalities in patients who presented with recurrent infections and were diagnosed with rare syndromes, with the goal of providing insights for treatment of such patients.
Material and methods
This retrospective analysis included 14 patients with complaints of recurrent infections defined by the Jeffrey Modell Foundation (http://www.info4pi.org/library/educational-materials/10-warning-signs), all of whom had been diagnosed with a rare syndrome in our department between January 2006 and August 2020. The study was approved by the Necmettin Erbakan University Meram Medical School Ethics Committee (Date: 12.17.2021/No: 2021/3542). The parents of all included patients provided written informed consent for participation in the study. The parents of 11 patients also provided written informed consent for the publication of identifiable images. How- ever, the parents of the other patients did not approve as they had some concerns about the publication of the images.
The patients were evaluated in terms of their demographic characteristics, age at complaint onset and diagnosis, family history, clinical features, genetic mutations, immunological laboratory tests, and the response to immunoglobulin replacement therapy (IGRT) during follow-up. Complete blood count was performed on the peripheral blood samples taken in EDTA tubes. Serum immunoglobulin levels were analyzed through nephelometry. The results were presented in mg/dl, and compared with age-appropriate normal values [4]. Serum anti-tetanus anti-HBs levels were analyzed with ELISA kits. Vaccine-induced blood levels of anti-HBs > 10 mIU/ml and anti-tetanus ≥ 0.1 IU/ml were considered protective. Isohemagglutinin titers are antibodies containing IgM against blood group antigens (anti-A and anti-B), and a titer of > 1/8 was considered sufficient for immunity.
Eight-color flow cytometer (BD FACSCanto II) was used for the analysis of peripheral blood lymphocyte subset, activation of CD25(+) T lymphocytes by mitogen (basal/72 hours), the percentage of class-switched B lymphocytes, and recent thymic emigrants (RTE). The absolute numbers of CD3(+), CD4(+) and CD8(+) T lymphocytes, CD3-16/56(+) NK cells were compared with age-appropriate normal values [5]. In addition, the percentage of class-switched B lymphocytes (CD19 + CD27 + IgD (–) (%)) and the percentage of RTE (CD4(+) CD31(+) CD45RA(+) (%)) were compared with age-appropriate normal values. In order to evaluate the functions of T-cells, activation of CD25(+) T lymphocytes by mitogen was maintained for 72 hours and compared with the basal level. Results below a 5-fold change were considered low. Diagnosis of the syndromes was performed at Necmettin Erbakan University, Medical Genetics Department.
Statistical analysis
SPSS for Windows version 25.0 (IBM Corp., Armonk, NY, USA) was used to evaluate the collected data. Continuous variables are expressed as means ± standard deviations or medians (ranges). Because the data exhibited skewed distributions, nonparametric tests were used. For comparisons of continuous variables, the Mann-Whitney U test was used. For comparisons of categorical variables, the chi-squared test was used. P-values below 0.05 were considered statistically significant in all analyses, and 95% confidence intervals were reported as appropriate.
Results
Our patients were diagnosed with Aicardi syndrome (P1), Brugada syndrome (P2), Phelan-McDermid syndrome (PMS) (P3), trichothiodystrophy (P4), LEOPARD syndrome (P5), Prader-Willi syndrome (P6), Seckel syndrome (P7), trisomy 18 (Edwards’ syndrome) (P8), Wiedemann-Steiner syndrome (P9), West syndrome (P10), Williams syndrome (P11), 47,XYY syndrome (P12), 16p13 deletion syndrome (P13), and 13q1.3 deletion syndrome (P14). Seven patients (50%) were girls. The patients’ demographic data are presented in Table 1, and photographs of the patients are shown in Figure 1.
Table 1
Diagnosis and follow-up data stratified according to sex
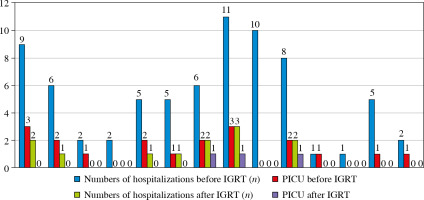
The mean current age was 56.7 ±32.9 months (median [range], 45.5 [27-153] months). There was a high rate of consanguinity (50%). Among the 14 patients, 10 (71%) were born via cesarean section; 5 of these patients (35.7%) were premature (Table 2). Four patients (28.5%) had deceased siblings. The sibling of the patient with Seckel syndrome also had a history of the syndrome; the other patients had no family history of their syndrome. There was one patient (P4) whose condition was inherited as autosomal recessive and related with consanguinity. We performed whole exome analysis in this patient (P4) and did not perform advanced genetic tests in the other patients after diagnosis. No patients had a family history of immunodeficiency. Body weight and height were below the 3rd percentile (< 3 P) in 50% of the patients, while 6 patients (42.8%) exhibited a head circumference below the 3rd percentile. Two patients had a head circumference above the 97th percentile (> 97 P). In four patients (28.5%), all measurements (weight, height, and head circumference) were < 3 P; in three patients (21.4%), all measurements were within normal ranges. Three patients (21.4%; P7, P8, and P10) were oxygen-dependent; they received oxygen using a portable O2 concentrator. Genetic evaluations according to clinical characteristics and laboratory analysis are shown in Table 2. In terms of infections, 13 patients (92.8%) had recurrent pneumonia, 5 (35.7%) had recurrent skin infection, 4 (28.5%) had recurrent moniliasis and otitis, and 3 (21.4%) had recurrent diarrhea (Table 2). Furthermore, 11 patients (78.5%) had been hospitalized in the intensive care unit at least once (Fig. 2). Trimethoprim-sulfamethoxazole was administered to six patients (42.8%), while acyclovir prophylaxis was administered to one patient.
Table 2
Clinical features on admission in patients with rare syndromes
[i] C/S – cesarean section, PM – prematurity, MN – moniliasis, PN – pneumonia, SI – skin infection, HK – herpes keratitis, EP – epilepsy, HT – hypertension, BW – body weight, HC – head circumference, FA – food allergy, HCM – hypertrophic cardiomyopathy, DCM – dilated cardiomyopathy, PH – pulmonary hypertension, O2 – oxygen-dependent, SVAS – supravalvular aortic stenosis, H – hypothyroidism, B – blindness, TX – trimethoprim/sulfamethoxazole
Immunological evaluations on admission revealed neutropenia in two patients (P4 and P12), and lymphopenia (P9) in one patient. Seven patients (50%) had low IgG levels (P3, P4, P6, P8, P9, P10, and P14), five (35.7%) had low IgA levels (P2, P5, P10, P11, and P12), five (35.7%) had low IgM levels (P3, P6, P8, P9, and P10), and one had panhypogammaglobulinemia (P10). Three patients had normal immunoglobulin levels with a reduced number of class-switched memory B lymphocytes, a reduced lymphocyte proliferation response to mitogen, and/or reduced specific antibody responses. In addition, two patients had elevated IgE levels (P4 and P7); this manifestation was accompanied by food allergy (Table 3).
Table 3
Immunological findings at admission in patients with rare syndromes
[i] N – normal, ALC – absolute lymphocyte count, ANC – absolute neutrophil count, Class-switched B lymphocyte: CD19+CD27+IgD(–), RTE (recent thymic emigrants): CD4+CD31+CD45RA+, CD3+ (%)/absolute counts (mm3), CD4+ (%)/absolute counts (mm3), CD8+ (%)/absolute counts (mm3), CD19+ (%), NK – natural killer (CD16+CD56+) (%), CD25 activation (basal/72 h), IH – isohemagglutinin, Tetanus A – tetanus antibody
Peripheral blood lymphocyte subset analysis showed that the percentages and absolute counts of CD3+ T lymphocytes were low in five patients (35.7%; P4, P5, P8, P9, and P13). The percentages and absolute counts of CD3+ CD4+ helper T lymphocytes were low in four patients (28.5%; P5, P8, P9, and P13); the percentages and absolute counts of CD3+ CD8+ cytotoxic T lymphocytes were low in five patients (35.7%; P3, P4, P5, P8, and P9). The percentages of CD16+ 56+ natural killer (NK) cells were low in six patients (42.8%; P2, P5, P6, P11, P12, and P14), and the percentage of CD19+ B lymphocytes was low in one patient (P9). Furthermore, a low NK cell count accompanied by neutropenia was observed in one patient (P12). Overall, 11 patients had lymphocyte subset abnormalities in their peripheral blood; the remaining 3 patients had normal lymphocyte subsets with abnormalities in either immunoglobulin levels or class-switched B lymphocyte ratios (Table 3).
The percentage of class-switched B lymphocytes was low in 11 patients (78.5%). In addition, evaluation of CD25 expression on T lymphocytes at 72 h after mitogen stimulation revealed low levels in 11 patients (78.5%), while the number of recent thymic emigrants was low in 5 patients (36%). Concerning antibody titers, the isohemagglutinin titer was measured in 10 patients; it was low in 8 of them (80%). The tetanus antibody titer was examined in 12 patients; it was low in 8 of them (66.6%). The HBs antibody titer was examined in 13 patients; it was low in 7 patients (53.8%) (Table 3). In addition, the ratio and number of CD45+RA and CD45+RO T lymphocytes were found to be normal.
All patients were administered IGRT (intravenous immunoglobulin therapy 0.4-0.8 mg/kg/3 weeks). The main criteria for IGRT were hospitalization for recurrent infections and the need for care in an intensive care unit for these conditions. During follow-up, no side effects were observed, with a median follow-up duration of 30.29 ±12.18 months. Neutropenia was persistent in two patients (P4 and P12). Furthermore, eight patients (57.1%) had low IgG levels (P3, P4, P6, P8, P9, P10, P11, and P14), six (42.8%) had low IgA levels (P2, P4, P5, P10, P11, and P12), seven (50%) had low IgM levels (P3, P6, P8, P9, P10, P12, and P14), and one (P10) had panhypogammaglobulinemia. In addition, two patients (P4 and P7) had elevated IgE levels. Peripheral lymphocyte subset analysis showed that the proportion of NK cells was low in two patients (P2 and P14) who otherwise had normal T-cell counts and functions. However, the proportion of class-switched B lymphocytes was low in 13 patients (92.8%). Concerning antibody titers, the isohemagglutinin titer was measured in 12 patients; it was low in 9 patients (75%).
Overall, all patients with rare syndromes in this cohort had abnormalities in the following characteristics on admission: immunoglobulin levels, lymphocyte subsets, CD25 expression, and specific antibody responses. During the follow-up after IGRT, all T-cell abnormalities were improved, while B-cell abnormalities persisted; these findings suggested that our patients predominantly had antibody deficiencies associated with mild T-cell abnormalities caused by recurrent infections. After IGRT, there were significant reductions in the number of infection- related hospitalizations (from 5.21 ±3.35 [median, 5] to 0.86 ±1.03 [median, 1]) and the number of intensive care unit admissions (from 1.36 ±1.01 [median, 1] and 0.21 ±0.43 [median, 0]) (p < 0.001) (Fig. 2). The proportion of patients < 3 P for body weight and/or height also decreased, to 28.5% (n = 4). Of the three oxygen-dependent patients, oxygen dependency was improved in two; only one patient with tracheostoma remained oxygen-dependent. There was no cardiological condition leading to hospitalization and recurrent infections in our patients. One of them (P13) has dilated cardiomyopathy and another (P5) has hypertrophic cardiomyopathy, and neither patient has been hospitalized during IGRT. IGRT was discontinued in two patients (P7 and P13) who exhibited significant clinical improvement during follow-up.
Discussion
This study evaluated the clinical and laboratory manifestations of immunodeficiency in patients with recurrent infections diagnosed with a rare syndrome. It also emphasized the effects of immunodeficiency in these patients, because such manifestations usually occur in early childhood; they are important contributions to morbidity and mortality. We found that the immunodeficiency was predominantly characterized by antibody deficiency in our patients with rare syndromes; clinical improvement was achieved via IGRT. We propose immunological evaluations for patients with recurrent infections; IGRT may improve the prognosis of such patients.
In our study, the mean age at complaint onset was 13.64 ±31.99 months, the mean age at diagnosis was 26.7 ±30.59 months, and the mean duration of delay was 13.1 ±11.0 months. In a case series of Turkish patients with primary immunodeficiency (PID), Yorulmaz et al. [6] reported that the mean age at diagnosis was 24.6 ±11.3 months and the mean duration of delay was 13.5 ±8.6 months; these findings were similar to those of our study. Previous studies showed that recurrent infections were present in patients with syndromic immunodeficiencies; these included respiratory tract infections, skin infections, otitis, and diarrhea [7, 8]. One study evaluated patients with chromosomal abnormalities and recurrent infections in terms of their immunological characteristics; PID was present in one-third of the patients [9]. Among our patients, 92.8% had recurrent pneumonia, 35.7% had recurrent skin infections, 28.5% had recurrent moniliasis and otitis, and 21.4% had recurrent diarrhea. Furthermore, 78.5% of our patients had been hospitalized due to infection at least once in the intensive care unit; all of our patients received a diagnosis of immunodeficiency after immunological evaluation. We suspect that some patients with rare syndromes have immunodeficiencies that lead to severe infections; a diagnosis of immunodeficiency might facilitate appropriate treatment for such patients.
Among our patients diagnosed with rare syndromes, some had a PID defined in the literature, while the others did not. Chromosomal abnormalities, particularly deletions (e.g., Di George syndrome, chromosome 11q deletion syndrome, Wolf-Hirschhorn syndrome, and deletion of the short or long arm of chromosome 18), are reportedly associated with primary immunodeficiencies, such as B- and T-cell deficiencies, and reduced numbers of class-switched B lymphocytes [1, 10, 11]. In our study, five patients had deletions [PMS (P3), Prader-Willi syndrome (P6), Williams syndrome (P11), 16p13 deletion (P13), and 13q21 deletion (P14)]; all of those patients had PID. Patients with PMS syndrome are prone to immune system disorders such as recurrent ear and upper respiratory tract infections, food allergies, and asthma. In addition, animal studies have demonstrated that a loss of function in the tip of chromosome 22 may influence immune function [12]. In 2017, Bogaert et al. described two siblings with recurrent respiratory tract infections; one was diagnosed with common variable immunodeficiency in early childhood and subsequently diagnosed with Wiedemann-Steiner syndrome [13]. Other reports have described chronic granulomatous disease in patients with Williams syndrome [14, 15]. Moreover, Seckel syndrome may cause PID involving deficiencies in B cells and phagocytes [1]. Patients with trichothiodystrophy may have hypogammaglobulinemia and neutropenia; they exhibit an increased risk of death before 10 years of age. In our study, the patients diagnosed with PMS (P3) and Wiedemann-Steiner syndrome (P9) had B- and T-cell defects on admission [16]. Additionally, the patient diagnosed with Williams syndrome (P11) had both B- and T-cell defects, as well as a low proportion of class-switched B lymphocytes; that patient’s dihydrorhodamine test results were normal. Our patient diagnosed with Seckel syndrome had a T-cell defect, together with a low proportion of class-switched B lymphocytes. Our patient diagnosed with trichothiodystrophy had neutropenia, B- and T-cell defects, and a low proportion of class-switched B lymphocytes.
Importantly, our patients diagnosed with Prader-Willi syndrome, 13q21 and 16p13 deletion syndromes, Aicardi syndrome, Brugada syndrome, West syndrome, LEOPARD syndrome, trisomy 18, and 47,XYY syndrome exhibited no immunodeficiencies that have been reported in the literature. The patients diagnosed with Prader-Willi syndrome and 13q21 deletion had a B-cell defect and mild T-cell functional defect on admission; they also exhibited low NK cell and class-switched B lymphocyte counts. Our patient with 16p13 deletion had B- and T-cell defects, as well as a low class-switched B lymphocyte count. Clinical management, follow-up, and treatment are generally similar for patients with LEOPARD syndrome and those with Noonan syndrome [17, 18]. However, some clinical manifestations in patients with LEOPARD syndrome require distinct management approaches. In our study, the patient diagnosed with LEOPARD syndrome had B- and T-cell defects on admission, along with a low proportion of class-switched B lymphocytes. The patient with trisomy 18 was tracheostomized and exhibited both epilepsy and hypothyroidism; immunologic examinations revealed B- and T-cell defects, as well as a low class-switched B lymphocyte count. The 47,XYY syndrome is a sex chromosome abnormality. Berglund et al. reported that pneumonia, asthma, and chronic obstructive pulmonary disease were more common in patients with 47,XYY syndrome than healthy controls [19]. Immunologic examination of our patient with 47,XYY syndrome revealed neutropenia, a B-cell defect, and a low proportion of class-switched B lymphocytes. The typical triad of Aicardi syndrome was first described in 1965; it comprises infantile spasms, chorioretinal lacunae, and agenesis of the corpus callosum [20]. On admission, our patient with Aicardi syndrome exhibited a B-cell defect and low class-switched B lymphocyte count. Brugada syndrome is a genetic disease that causes greater vulnerability to fatal cardiac arrhythmias [21]. Our patient with Brugada syndrome had a B-cell defect, along with low NK cell and class-switched B lymphocyte counts. Moreover, immunologic examination of our patient with West syndrome demonstrated B- and T-cell defects, as well as a low class-switched B lymphocyte count. For patients with a history of recurrent infections and syndromic disorders, consideration of immunodeficiencies in the differential diagnosis and prioritization of immunological evaluations would facilitate early diagnosis of the causative diseases.
In our study, some patients had reduced B-cell or T-cell counts on admission; in some patients, both lineages were affected. All patients were administered IGRT; six patients received trimethoprim-sulfamethoxazole prophylaxis and one received acyclovir prophylaxis. During follow-up, B- and T-cell counts became normalized; all patients had transient or prolonged hypogammaglobulinemia. In addition, the rate of infection-related hospitalization decreased after IGRT, and the rate of intensive care unit admissions also considerably decreased. Among oxygen-dependent patients, the need for oxygen decreased, such that only one tracheostomized patient remained oxygen-dependent. These findings suggested that our patients predominantly had antibody deficiency, and there was also an association with mild T-cell abnormalities because of recurrent infections.
Importantly, consanguinity and cesarean section were comorbidities in our patients with rare syndromes. A high rate of consanguineous marriages carries a substantial risk of syndromic immunodeficiencies because consanguinity increases the risks of autosomal recessive and multifactorial diseases [6, 22]. In Asia, Africa, and Middle East, the rate of consanguineous marriages is high (20-70%) due to sociocultural factors [6, 23-25]. In our series, the rate of consanguinity was high (50%) compared with the general rate of such marriages in Turkey (25%) [6, 22, 25]. This high rate may have been associated with the onset of syndromic immunodeficiency in our patients. In general, cesarean deliveries are performed when there are significant risks of maternal and/or fetal morbidity and mortality with vaginal delivery. The indications for cesarean delivery include cephalopelvic disproportion, fetal anomalies, and congenital anomalies [26]. In 2009, 32.9% of all births in the USA were performed via cesarean section [27]. Premature births constitute 9-12% of all births worldwide; such births are the leading cause of neonatal morbidity and mortality [28]. Among our patients, 71% were born via cesarean section and 35.7% had comorbid prematurity. The high rates of cesarean section and prematurity may have contributed to the onset of rare syndromes in our patients.
The limitations of this study were that these patients with a rare syndrome were heterogeneous and the relation between immunodeficiency and chromosomal abnormalities was not studied. We believe that certain chromosomal abnormalities might be related to possible immune mechanisms regarding the humoral and cellular immune system, and further functional and epigenetic studies are needed to investigate this association.
In conclusion, some of the syndromes associated with immunodeficiency in this study have not been reported in the literature and patients with rare syndromes might have immunological abnormalities. Our findings indicate that immunological abnormalities and recurrent infections in patients with rare syndromes might be improved with IGRT. Our findings also support the use of IGRT for patients with early onset immunological disorders, as it can improve the prognoses of such patients.