Purpose
Ocular melanoma (OM) accounts for 2.9% of all melanomas in the United States and is the most common primary intraocular malignancy in adults [1]. Approximately, 95% of OM arise from uveal melanocytes, including iris, ciliary body, and choroid. The majority arise from the choroid and ciliary body (90-95%), which are more likely to be fatal than tumors in the iris [2]. As such, tumors of the choroid and ciliary body are treated more aggressively and have been treated historically with enucleation.
In recent decades, studies have been focusing on adopting treatments with the potential for globe perseveration without compromising survival. Among those treatments is eye plaque brachytherapy (EPBT), where a plaque with radioactive sources is surgically implanted on the sclera overlying the tumor and subsequently removed, following dose delivery. The Collaborative Ocular Melanoma Study (COMS) group conducted a phase III randomized trial that demonstrated medical equipoise between patients with medium sized OM treated with either enucleation or EPBT, with no survival differences found between the treatment cohorts [3]. Overall survival was about 81% at 5 years and 57% at 12 years in both arms [4]. The inclusion criteria were defined as medium sized tumors, with height between 2.5-10 mm and diameter less than or equal to 16 mm. This study established eye plaque brachytherapy as a standard of care for patients with medium sized choroidal melanoma tumors.
The National Comprehensive Cancer Network (NCCN) recommendations include particle beam radiotherapy, stereotactic radiosurgery (SRS), or enucleation for large sized tumors (diameter > 18 mm, height > 10 mm) [5]. EPBT is not listed as a treatment option for large size tumors, but it is for small size tumors (diameter < 18 mm, height < 2.5 mm) and medium (diameter < 18 mm, height 2.5-10 mm). The American Brachytherapy Society (ABS) and the American Joint Committee on Cancer (AJCC) guidelines recommendations state T1, T2, T3, and T4a-d uveal melanoma patients can be treated, after counseling about likely vision, eye retention, and local control outcomes. The ABS guidelines list contraindications to EPBT include large size tumors with extraocular extension (AJCC T4e), basal diameters that exceed the limits of brachytherapy, blind painful eyes, and those with no light perception vision [6]. Retrospective and prospective cohort studies have demonstrated acceptable local control, with 87-94% for large sized OM tumors treated with EPBT [7,8].
There is no current randomized data comparing the efficacy of brachytherapy and enucleation for patients with large tumors. Based on current improvements in technology for delivery of EPBT, we hypothesized that the use of EPBT would result in equivalent survival outcomes for larger sized tumors, which was the impetus for the current study [9]. The purpose of the present study was to investigate current practice patterns and compare survival outcomes between different treatment options for patients with choroidal melanoma of all sizes using a large, contemporary database.
Material and methods
The National Cancer Database (NCDB) is a national hospital-based cancer registry that is co-sponsored by the American College of Surgeons (ACoS) and the American Cancer Society. The NCDB collects data from more than 1,500 hospitals with ACoS-accredited cancer programs, accounting for 70% of all newly diagnosed cancers in the United States [10,11,12,13]. The most recent data from the NCDB included records from 2004 to 2014. Institutional review board approval was not required for this study, as only de-identified information is stored in the database.
For this study, the NCDB was queried for histologically-confirmed choroidal or ciliary body melanoma for patients ≥ 18 years old. Inclusion criteria consisted of patients with N0 disease, M0 disease, those who have OM as their first and only malignancy, and those who have a known vital status (Figure 1). Patients were divided into two cohorts: those treated with brachytherapy alone versus enucleation alone. While EPBT is commonly used, the NCDB does not specifically mention the exact type of brachytherapy device. Demographic and clinical data included age, sex, treatment facility type, treatment year, race, insurance status, Charlson/Deyo comorbidities score, distance to treatment, time to starting radiation, and medium income of the patient’s home zip code were included [14]. The Charlson/Deyo comorbidities was applied, since it is the only such score used by the NCDB. Some patients were missing either staging information or tumor dimension measurements. In order to minimize biases introduced by complete case analyses, patients with tumor dimension information only or those who have tumor dimension information and staged using the American Joint Committee on Cancer (AJCC) 7th edition, were restaged using the AJCC 6th edition. This approach was employed as this staging scheme more closely resembled size criteria used in the COMS trials and NCCN that allows for the inclusion of largest proportion of patients. Patients were divided into cohorts representing small, medium, and large tumors (Table 1). For patients that had both tumor size and staging information, the highest level was used. Ciliary body invasion (CBI) or extraocular extension (EOE) were also evaluated.
Fig. 1
Consort diagram
EPBT – eye plaque brachytherapy, C69.3 – malignant neoplasm of choroid, C69.4 – malignant neoplasm of ciliary body
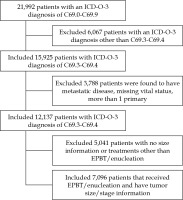
Table 1
Size definitions comparing COMS size and the AJCC 6th edition
All statistical tests were two-sided, with a threshold p-value lower than 0.05 for statistical significance, and were performed using SPSS Statistics, version 23 (IBM Corporation, Armonk, NY, USA). The χ2 test were used to compare categorized demographic and clinical variables in both arms. The Kaplan-Meier method was applied for survival analysis, and comparisons between the two treatment paradigms were performed with the log-rank test for all patients. Overall survival (OS) was defined as the interval between the date of diagnosis and the date of death from any cause or last contact. OS is the only disease-related outcome listed in the NCDB. Multivariable Cox proportional hazards modeling was additionally employed to identify variables associated with OS in the entire cohort. Propensity score matching (PSM) was conducted, which used propensity scores based on differences in demographic and clinical variables (age, comorbidity score, treatment distance, facility, education level, insurance, income level, sex, race, city, tumor size, and extraocular extension), and a 1 : 1 matched cohort of brachytherapy and enucleation patients were created using the MatchIt function in R, version 3.5.2 (the R Foundation for Statistical Computing, 2015) [15]. The matched cohorts of 3,190 patients (1,595 in each group) were separately analyzed with the Kaplan-Meier and Cox proportional hazards analyses in the fashion described above.
Results
A total of 7,096 patients met the inclusion criteria, approximately 35% of patients treated in the USA in this time period; 5,501 (78%) patients received brachytherapy and 1,595 (22%) of patients were treated with enucleation (Figure 1) [16]. Median follow-up was 55 and 38 months for brachytherapy and enucleation, respectively. Brachytherapy patients were more likely to be from educated zip codes, have private insurance, live farther away from where they were treated, and have a higher Charlson/Deyo comorbidities score (Table 2). Enucleation patients were more likely to be from low-income zip codes, have government insurance, be uninsured, have EOE, have CBI, and have large size tumors (Table 2).
Table 2
Demographic data comparison for all patients
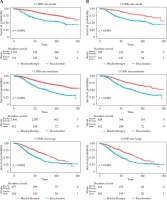
Amongst all patients, 5-year overall survival (5-yr OS) was 80% vs. 54% for brachytherapy and enucleation, respectively (p < 0.001) (Table 3). 5-yr OS for small tumors was 86% vs. 64%, for medium tumors was 80% vs. 57%, and for large tumors was 67% vs. 46% for brachytherapy and enucleation, respectively (p < 0.001) (Table 4) Kaplan-Meier curves show survival trend for all patients divided into size cohorts (Figure 2A).
Table 3
5-year overall survival (OS) for all patients
| Treatment | n patients | Mean follow-up (months) | Median follow-up (months) | Range | 5-year OS |
|---|---|---|---|---|---|
| Brachytherapy | 5,501 | 61 | 55 | 0-156 | 80.0% |
| Enucleation | 1,595 | 48 | 38 | 0-153 | 54.0% |
| Total | 7,096 | – | – | – | p < 0.001 |
Table 4
5-year overall survival (OS) for patients divided by COMS size for all patients
| COMS size | Brachytherapy cohort | Enucleation cohort | Log rank | ||
|---|---|---|---|---|---|
| n patients | 5-year OS | n patients | 5-year OS | ||
| Small | 1,183 | 86.4% | 331 | 64.1% | p < 0.001 |
| Medium | 3,641 | 80.2% | 612 | 56.6% | p < 0.001 |
| Large | 677 | 67.3% | 652 | 45.7% | p < 0.001 |
After propensity matching, the treatment cohorts had no difference in demographic/clinical variables, except for more EOE in the enucleation cohort (15% vs. 10%, p < 0.001) (Table 5). 5-yr OS was 76% vs. 54% for brachytherapy and enucleation, respectively (p < 0.00) (Table 6). 5-yr OS for small tumors was 87% vs. 64%, for medium tumors was 77% vs. 57%, and for large tumors was 68% vs. 46% for brachytherapy and enucleation, respectively (p < 0.001) (Table 7). Kaplan-Meier curves show survival trend after PSM divided into size cohorts (Figure 2B).
Following PSM, the multivariate Cox regression modeling found older age (hazard ratio [HR] = 1.76, 95% confidence interval [CI] = 1.51-2.06), more comorbidities (HR = 1.46, 95% CI = 1.25-1.70), EOE (HR = 1.25, 95% CI = 1.06-1.48), CBI (HR = 1.20, 95% CI = 1.02-1.40), and larger tumors (HR = 1.52, 95% CI = 1.40-1.66) as negative prognosticators of survival. Brachytherapy was a positive prognosticator of survival (HR = 0.45, 95% CI = 0.40-0.51) (Table 8).
Table 5
Demographic data comparison after PSM
Table 6
5-year overall survival (OS) for all patients after propensity score matching
| Treatment | n patients | Mean follow-up (months) | Median follow-up (months) | Range | 5-year OS |
|---|---|---|---|---|---|
| Brachytherapy | 1,595 | 59 | 53 | 0-152 | 75.8% |
| Enucleation | 1,595 | 48 | 38 | 0-153 | 54.0% |
| Total | 3,190 | – | – | – | p < 0.001 |
Table 7
5-year overall survival (OS) for patients divided by COMS size after propensity score matching
| COMS size | Brachytherapy cohort | Enucleation cohort | Log rank | ||
|---|---|---|---|---|---|
| n patients | 5-year OS | n patients | 5-year OS | ||
| Small | 355 | 86.5% | 331 | 64.1% | p < 0.001 |
| Medium | 628 | 77.1% | 612 | 56.6% | p < 0.001 |
| Large | 612 | 67.9% | 652 | 45.7% | p < 0.001 |
Table 8
Cox proportional hazards multivariate model after propensity score matching
Discussion
In this large retrospective review, patients selected for brachytherapy had improved survival compared to enucleation in all size cohorts. This result persisted after propensity score matching. Given that the COMS medium trial was a prospective randomized trial showing equivalence of brachytherapy and enucleation for medium sized tumors, the superior OS for brachytherapy seen in our study was very likely due to selection bias in the data, as the patients who underwent enucleation were more likely to be uninsured, with a lack of financial resources, and having tumors too large to be amendable to EPBT [3]. Patients with large tumors selected for EPBT were likely to have smaller tumors on average than those who received enucleation. Since randomized evidence in large size tumors is lacking, our data can help to provide evidence for clinicians that brachytherapy is a reasonable treatment option for selected patients with large size tumors. EOE and CBI were significantly higher in the enucleation cohort and were likely to bias the clinician against treating with brachytherapy.
Mortality from ocular melanoma was initially believed by some to be from metastatic seeding at the time of enucleation, which was known as the “Zimmerman hypothesis” [17]. The COMS trial involving large size OM included patients with tumor height > 10 mm or diameter > 16 mm randomized to either external beam radiation (20 Gy in 5 fractions) before enucleation versus enucleation alone [18]. The concept for this approach was that pre-enucleation radiation could sterilize the area surrounding the tumor bed, decreasing the chance of metastatic seeding from the enucleation procedure. No significant difference was demonstrated for all-cause mortality or distant metastases at 5-year and 10-year follow-up. The findings disproved Zimmerman’s hypothesis, suggesting that metastatic spread occurred prior to enucleation or other local treatment.
Recent studies use a 12-gene expression profiling (GEP) assay to classify the aggressiveness of OM tumors [19]. They are divided into 1A, 1B, and 2 classes, with associated 5-year metastatic risk of 2%, 21%, and 72%, respectively. In the highest risk group, class 2, OM tumors are associated with larger tumor dimensions as well as GEP. Other high-risk features, such as CBI and EOE are associated with cytogenetic abnormalities [20]. Therefore, modern genomics has confirmed that the majority, if not all, of patients who undergo local therapy and then develop clinically evident metastases at a delayed time to have non-radiographically detectable micrometastases present at the time of diagnosis. Thus, which procedure is chosen for local control has only a marginal effect on survival outcomes. The COMS natural history study also confirmed this theory [21]. As such, EPBT may be a preferable treatment for these tumors since it allows for globe and vision preservation.
EPBT is an established treatment for small and medium sized tumors as discussed above. Retrospective studies and prospective case series regarding EPBT for large size tumors have demonstrated low rates of local recurrence, 6-9% at 5 years, which is comparable to COMS medium trial with 10.3% at 5 years (Table 9) [3,4,7,8,22]. The rates of metastasis mortality, 30-35% at 5 years, are comparable to COMS large trial with 28% at 5 years. Overall survival, 62% at 5 years in a retrospective paper, is in between COMS medium, 81% at 5 years, and COMS large trial, 57% at 5 years [8]. The 5-yr OS of 67.9% for large tumors after PSM in our data is comparable to the 5-yr OS found in the retrospective paper.
Table 9
Comparison of papers regarding eye plaque brachytherapy used to treat large size tumors to COMS trials
The retrospective nature of this analysis is recognized as a limitation. While the NCDB only represents a percentage of patients treated in the United States, it has benefits over surveillance, epidemiology, and end results program (SEER) database, since it includes important clinical factors, such as tumor depth and size as well as socioeconomic factors [23]. However, the NCDB has additional limitations, including a lack of information regarding the gene expression profile of the tumors. There is a lack of information regarding local control, metastasis mortality, dose for brachytherapy, and toxicity. Potential adverse events from EPBT include scleral necrosis, strabismus, cataract, glaucoma, and retinopathies [24]. While PSM was used to reduce selection bias and potential clinical or demographic cofounders, even this analysis cannot completely eliminate confounding biases. Further prospective studies are warranted to determine criteria to select patients with large size tumors for brachytherapy to increase eye preservation rates.
Conclusions
Ocular melanoma is a rare disease with worse survival than other melanomas. Tumor genetics, size, and early micrometastatic spread are the most important indicators for survival. Therefore, local treatments other than enucleation, such as eye plaque brachytherapy, should be safe to use to preserve a patient’s vision and aesthetics. To our knowledge, this study is the largest study of eye plaque brachytherapy versus enucleation, and our findings suggest that EPBT is a reasonable treatment option for all size ocular melanoma tumors, including large size tumors.