Purpose
Inoperable primary or secondary tumors (metastases) are frequently treated with interstitial brachytherapy, which allows for application of high radiation doses to a lesion, with a steep dose fall-off outside of the target and sparing surrounding (healthy) tissue [1,2,3,4]. Multiple brachytherapy treatment modalities are available, including catheter-based high-dose-rate (HDR) afterloading brachytherapy with 192Ir sources or permanent implantation with 125I seeds (PSI), as the most used methods [5].
An emerging technical alternative may be electronic brachytherapy (EBT) with miniaturized linear accelerators that generate low-energy X-rays, which are rapidly absorbed in surrounding tissue, showing dose gradients similar to PSI and steeper than in HDR [6]. In contrast to HDR and PSI, EBT does not require radionuclides, source replacements, or post-interventional precautions. Additionally, EBT devices are mobile and may be used in any room that fulfils basic requirements for operating kV-energy emitting X-ray devices (e.g., classical X-ray imaging, C-arm fluoroscopy, or computed tomography – CT) [7,8,9]. Current applications of EBT mostly involve intracavitary (intraoperative) radiotherapy of breast, brain, and spine tumors, but also intravaginal or intrauterine EBT and superficial EBT of skin tumors [10,11, 12,13,14,15,16].
To evaluate whether EBT is a feasible alternative to contemporary standard technology, we created radiation treatment plans with stepping source EBT in post-implantation CT scans of patients treated with PSI, and assessed the conformality, doses covering 90% of the target volume (D90), and required trajectories in comparison to PSI as well as estimated treatment times for EBT.
Material and methods
Data set
Post-implantation CT data sets (DICOM files; Brillance CT Big Bore, Koninklijke Philips N.V., Amsterdam, The Netherlands; resolution: 512 × 512, field of view: 600 mm × 600 mm, slice thickness: 1 mm) of 10 patients (n = 10) that received CT-guided PSI (125I seeds, Eckert & Ziegler BEBIG GmbH, Berlin, Germany; activity at implantation between 0.43 mCi and 0.66 mCi, IPSA planning based on the TG-43 formalism with Oncentra prostate versions 4.0.7-4.2.2.4, Elekta AB, Stockholm, Sweden) alone or as a boost in combination with external beam radiotherapy (EBRT) to inoperable tumors progressing on standard therapy were chosen as templates for EBT treatment planning. This cohort represented all patients treated with CT-guided PSI for inoperable interstitial tumors from 2012 to 2019 in our department. All analyses were performed after approval of the institutional review board.
Target volumes and dose prescription
If a patient had received therapy with PSI only, the same planning target volume (PTV) was used for the simulation of EBT. If PSI had been combined with EBRT, the intent was either to boost the whole tumor or to treat parts of the tumor before EBRT (e.g., when parts of the tumor were in close proximity to organs at risk [OARs]). In case of partial treatment with PSI, the PTV still resembled the whole tumor and no dedicated partial PTV was done to treat as much of the PTV as possible with PSI. To establish a realistic partial PTV for simulation of EBT ex post, the 100%-isodose line of PSI was transformed into a structure in Velocity 3.2.1 (Varian Medical Systems, Palo Alto, CA, USA), and was considered as PTV. Subsequently, this partial PTV from PSI represented the PTV for EBT. For relative comparison only, previously prescribed PSI doses were adopted for EBT and required to cover 90% of the PTV for EBT.
EBT system
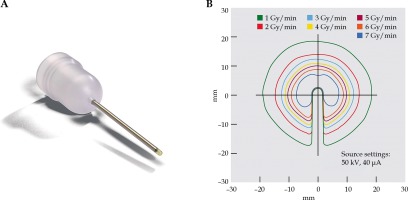
Electronic brachytherapy treatment was simulated using the Intrabeam system (Carl Zeiss Meditec AG, Oberkochen, Germany), with a needle-shaped applicator, which allowed for a spherical delivery of low-energy X-rays (maximum energy, 50 kV) emitted from the tip of applicator with a steep dose gradient (Figure 1) [17].
EBT treatment planning
Computed tomography studies and structure files (PTVs, OARs) were imported into Radiance 4.0.3/4.0.5 (GMV SA, Madrid, Spain). First, interstitial trajectories were chosen according to all requirements of a realistic needle placement (avoidance of organs, major vessels, nerves, and bones). Then, dwell positions were defined in 10 mm increments along the defined trajectories. Next, dose distributions were calculated multiple times for every dwell position, each time with differing prescription radii, using a non-TG-43 model-based dose calculation in a hybrid form of Monte Carlo-based algorithm for the distribution of kV-photons in heterogeneous tissue [18,19]. For dwell position dose summation, the CT studies and multiple dose files for each dwell position were exported to Velocity. By manual forward planning, a combination of dwell position doses was selected to generate a sum dose with the most conformal coverage of the PTV by the D90 of prescription dose stemming from PSI. To obtain conformality, the refinement of dwell positions along a trajectory was performed by increasing or decreasing the distance between dwell positions. Subsequently, dwell position dose calculation and forward planning were recommenced.
Plan evaluation
For each case, the sum dose of EBT to 90% of the PTV (D90 of EBT) was compared to the prescribed dose of EBT (adopted from PSI) and to originally reached D90 by PSI. If the D90 of EBT met the prescribed dose stemming from PSI, or if the D90 of EBT was higher than the D90 achieved with PSI, conformality was evaluated. Prior to conformality assessment, virtual dose distribution was corrected if dose accumulation in disruptive elements outside of the PTV occurred, including urethral catheters or metal implants as well as dose distribution outside the patient (dose to air). By determining the size of PTV, the volume of tissue enclosed by the isodose line of prescribed dose and the volume of PTV covered by the prescribed dose (V100), the conformation number (CN) was calculated [20]. If OARs were affected by the isodose line of prescribed dose, conformality was assessed with the conformal index (COIN) by multiplying the CN with an additional term, representing the fractions of OARs receiving the prescribed dose or more [21]. Only plans with values of CN or COIN greater than 0.6 were accepted as conformal [20]. If the D90 of EBT was lower than the prescribed dose or the D90 of PSI, or if conformality was not reached, the planning was repeated with alterations to dwell positions.
Irradiation time
For the estimation of beam-on time during the treatment, all acquired conformal sum doses were virtually standardized to 13 Gy by adjusting the dwell position doses in relation to each other. Here, 13 Gy represents a single-fraction dose for interstitial brachytherapy, corresponding to an equivalent dose in 2 Gy fractions (EQD2 = n × D × ((d + α/β)/(2 + α/β))) between 32.5 and 54 Gy (for an α/β-ratio between 3 and 10), considering the increased relative biological effectiveness (RBE) of 1.3 for low-energy kV-photons [2,22,23,24].
Results
Patients’ characteristics
This planning study was conducted using CT scans of 10 cases, of which 5 patients obtained PSI to treat local (or loco-regional) tumor recurrence, and 5 patients received PSI to palliate symptoms of metastases. Six of the patients were females and 4 males, and the average age at PSI treatment was 62.5 years (standard deviation [SD] = 11.6). The entities of tumors and further details are presented in Table 1.
Table 1
Patients’ characteristics and results of simulated interstitial EBT treatment. Table consists of underlying diseases, locations of the tumors treated, planning target volume for electronic brachytherapy (PTVEB), number of trajectories and dwell points, conformation number (CN) and conformal index (COIN), and beam- on time for a standardized dose of 13 Gy
Planning
Doses were adopted from PSI and prescribed to 90% of the PTV for EBT, with a minimum of 10 Gy, a maximum of 60 Gy, and an average of 31 Gy (SD = 14.6). The average volume of PTV was 50.6 cm3 (SD = 34.5), with a minimum of 11.9 cm3 and a maximum of 106.7 cm3. The total number of iodine seeds implanted during PSI ranged between 5 and 28, with an average of 15 iodine seeds (SD = 7.6), which required between 4 and 11 needle trajectories (average, 6.9, SD = 2.2). For EBT, the total number of trajectories per case varied between 1 and 13, with an average of 5.0 (SD = 3.2). All trajectories for EBT fulfilled requirements of a realistic puncture. In comparison to PSI, the number of trajectories needed for EBT was lower in 7 cases and the same in 2 cases. In 1 case, additional trajectories were necessary for EBT. Overall significance for less trajectories in EBT could not be shown in this cohort (Mann-Whitney U test, two-tailed, significance at p ≤ 0.05, U-value = 25, Z-score = 1.85203, p-value = 0.06432). The number of utilized dwell positions for EBT ranged from 2 to 25, with a mean of 10.9 (SD = 7.1) dwell positions per case. On average, 2.1 (SD = 1.1) dwell positions per trajectory were generated, with a minimum of 1 and a maximum of 4 dwell positions. An example of the simulated needle applicator stepping along a trajectory is shown in Figure 2. On average, total forward planning time per case was 3.0 h (SD = 1.4), with a range from 0.8 h to 5.7 h. Moreover, planning time strongly correlated with the number of trajectories and dwell positions (Pearson’s correlation coefficient, r (8) = 0.94, p < 0.05, and r (8) = 0.98, p < 0.05).
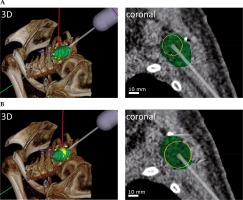
Fig. 2
Simulation of the needle applicator stepping along a virtual trajectory in a lesion in the left shoulder region. The PTV (green) contains two dwell positions, with (A) dwell position 1 and (B) dwell position 2, each displayed in 3-dimensionally rendered view (3D) and in a coronal plane. The dose distributions of dwell positions are displayed as isodose lines of 90%, 95%, and 100% of the prescribed dose in yellow, orange, and red, respectively
Coverage, conformality, and beam-on time
For all cases of simulated EBT, either the prescribed dose or a dose greater than the D90 obtained by PSI was reached. In 8 cases, the D90 acquired with EBT met the prescribed dose, exceeding it by an average of 4.7% (SD = 3.9), with a maximum of 12.6%. The prescribed D90 was not achieved in 2 cases, but still exceeded the D90 obtained with PSI (average, 21.3%, SD = 20.1) relative to the prescribed dose. The CN or COIN, respectively, were greater than 0.6 in all 10 cases, with values between 0.62 and 0.89, and an average of 0.69 (SD = 0.075). Two examples of conformal dose coverage of the PTVEBT by EBT are shown in Figure 3. The mean beam-on treatment time for EBT with the standardized dose of 13 Gy was 27.8 min (SD = 15.6).
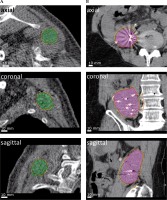
Fig. 3
Dose coverage after dwell position summation. Shown are two exemplary cases. A) For the first case (also displayed in Figure 2), a CN of 0.89 was achieved for a PTV in the left shoulder region (green). B) For the second case, a COIN of 0.63 was achieved for a PTV in the retroperitoneal space (pink). Isodose lines of 90%, 95%, and 100% of the prescribed dose displayed in yellow, orange, and red, respectively
Discussion
Here, for the first time, we demonstrated that stepping-source EBT may pose as a technical alternative to PSI for interstitial treatment of inoperable tumors, offering conformality but requiring less trajectories in most of our cases. EBT consistently achieved CN or COIN index values > 0.6, which is an accepted cut-off for a conformal dose distribution [20,21]. Although PSI and EBT have fundamental differences in dose rate and biological effectiveness, the nominal doses of PSI for relative comparison and assessment were prescribed [23]. As for dose adoption, up- or downscaling of the sum dose after the completion of EBT forward planning is facile, since dose is a function time in EBT and dwell position doses can be adapted in relation to each other to reach a desired sum dose. In a clinical setting, the dose prescription for EBT need to be individualized, depending on the overall treatment intention (curative or palliative), α/β-ratio of the tumor, and tolerance doses of surrounding tissues or organs. Moreover, the overall beam-on time mainly depends on the size of PTV, tissue density, and desired dose. When estimating the beam-on time with a standardized dose of 13 Gy, we never exceeded 30 minutes. Presumably, the prescription dose would considerably be lower in cases with palliative intent or due to OARs constraint, resulting in even shorter beam-on time.
A limitation of our study can be seen in the rigid registration of post-interventional CT scans, which did not allow for simulation of tissue deformation caused by needle applicator when calculating the dose distribution. Furthermore, these images also contained titanium-encapsulated seeds, which locally influence the dose distribution of EBT in the PTV. A particularity of EBT and iodine seeds is the absorption property of low-energy X-rays, with dose gradients of up to 50% per millimeter in close proximity to the applicator, provoking an inhomogeneity in the dose distribution with parts of the PTV receiving substantially more than 100% of the prescribed dose [25,26]. A greater number of dwell positions could mitigate these hot spots, but the treatment of lesions that contain radiation-sensitive structures should be cautiously evaluated. On the other hand, the sharp dose gradient of low-energy X-rays allows for effective sparing of tissues around the PTV. A special attention was paid to trajectory planning, as the Intrabeam system used for this EBT simulation requires a low number of trajectories due to diameter of needle applicator (4.2 mm) to avoid excessive tissue trauma, and limits the lesion depth with its needle length of 94 mm.
The number of necessary trajectories, with acquired level of conformality and acceptable beam-on time would allow for a transfer of stepping-source EBT into a clinical workflow, ideally as a CT-guided intervention. For an efficient procedure, a software algorithm for inverse planning with trajectory and dwell position optimization, as available for HDR and PSI (e.g., IPSA and HIPO) should be implemented [27,28]. Since duration of forward planning in this study was strongly correlated with the number of trajectories and dwell positions, it seemed feasible only for small lesions or lesions with favorable geometry (spherical). Similar to HDR or PSI, a workflow would start with CT-based target volume definition, dose prescription, and trajectory/dwell position calculation, followed by a suggestion of suitable trajectories. The puncture procedure would then consist of introducer needle or Kirschner wire and subsequent dilation, until a guidance tube could be placed to steer the needle applicator along the trajectory to the dwell positions. Strategies to move the needle applicator within the guidance tube already exist and include stereotactic frames or robotic guidance and can be combined with motion sensing and optical tracking [29,30,31].
Conclusions
Interstitial irradiation of inoperable lesions with needle applicator-based stepping-source EBT allow for conformal PTV coverage with the prescribed doses. Adaption of the prescription dose utilizing an algorithm for kV-photon distribution in heterogeneous tissue is feasible. Forward planning is achievable for small lesions but should be discarded in favor of inverse planning for larger PTV. The results of this planning study warrant the development of a clinical workflow.